Application of utilizing beta-phenylalanine compounds as aldose reductase inhibitors
A reductase inhibitor, phenylalanine technology, applied in the field of application of β-phenylalanine compounds as aldose reductase inhibitors, can solve problems such as side effects and poor selectivity, and achieve easy synthesis and selection High toxicity, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
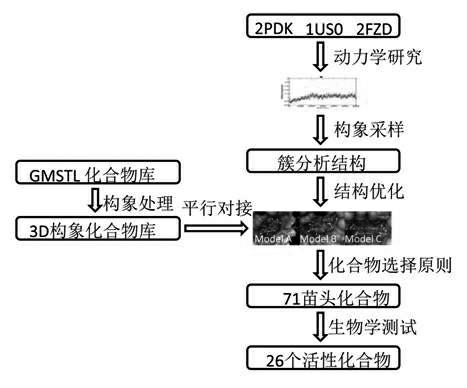
[0034] Example 1: Virtual Screening
[0035] The specific steps of virtual screening are as follows: figure 1 shown.
[0036] The crystal structures of three aldose reductases with three different binding pockets, which can represent all inhibitor binding pockets, were obtained from the protein crystal structure database. The codes of the proteins are 2PDK, 1US0, and 2FZD. Three crystal structures were processed as follows: water molecules were removed and missing amino acid residues were repaired. Kinetic studies were performed using these three structures as initial structures. Kinetic software uses molecular simulation software package AMBER10 to carry out. The amber molecular force field of FF03 is given to the protein, the GAFF force field is used for the small molecule, and the charge of the small molecule is fitted by the RESP method. Sampling analysis is done after the kinetics is completed, and the cluster analysis method is used to find a reasonable conformation ...
Embodiment 2
[0043] Example 2: Inhibition Test of Aldose Reductase
[0044] Using glyceraldehyde as a substrate, redox reaction occurs under the action of aldose reductase and coenzyme (NADPH), and NADPH is converted into NADP+, which shows the principle that the detectable absorption peak at 340nM in the ultraviolet region decreases. By changing the concentration of the compound to be tested , Measure the absorption peaks of different NADP+ obtained by the catalyzed substrate of aldose reductase after the action of different concentrations of drugs to judge the degree of inhibition of the drug on the enzyme, thereby calculating the IC50 value of the drug on the inhibition of aldose reductase.
[0045] The experiment uses recombinant human aldose reductase, which is obtained through cloning, expression, purification and testing. Glyceraldehyde and NADPH used in the experiment were purchased from Sigma Company. The compounds tested in the experiment were provided by our laboratory. The ex...
Embodiment 3
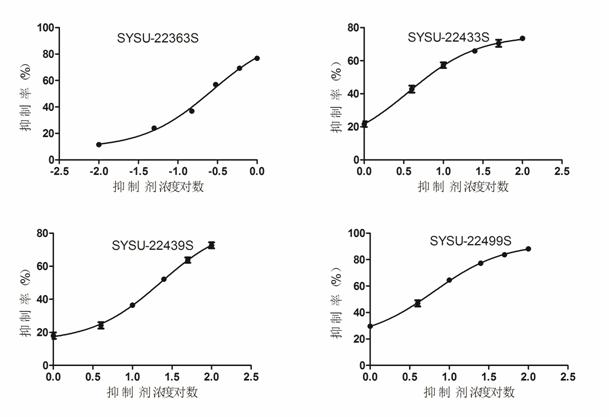
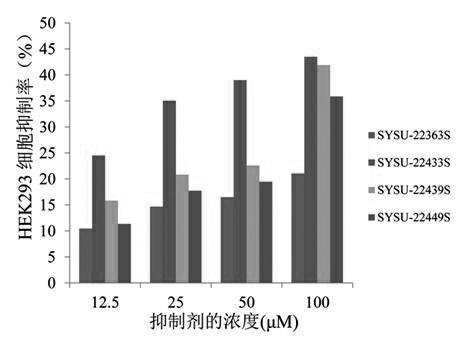
[0059] Example 3: Selective Inhibition Test of Aldehyde Reductase
[0060] Using glyceraldehyde as a substrate, a redox reaction occurs under the action of aldehyde reductase (ALR1) and coenzyme (NADPH), and NADPH is converted into NADP+, which shows the principle that the detectable absorption peak at 340nM in the ultraviolet region decreases. By changing SYSU- 22363S, SYSU-22433S, SYSU-22439S, SYSU-22449S four compound concentrations, measured the substrates of aldose reductase catalyzed by different concentrations of drugs and obtained different NADP+ absorption peaks to judge the degree of inhibition of the drug on the enzyme, and thus calculated IC50 values of drugs for inhibition of aldehyde reductase. The reaction system is similar to the aldose reductase system, the differences are as follows: no lithium sulfate is added to the system, the test temperature of the system is 37° C., and the concentration of aldehyde reductase used in the system is half of that of aldos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com