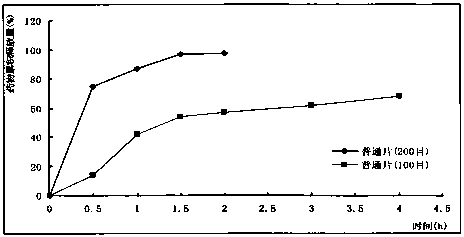
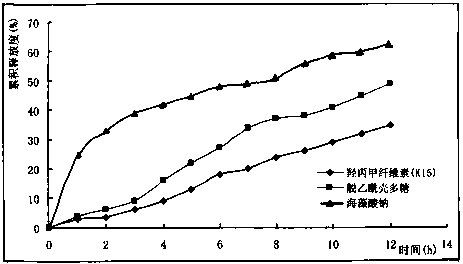
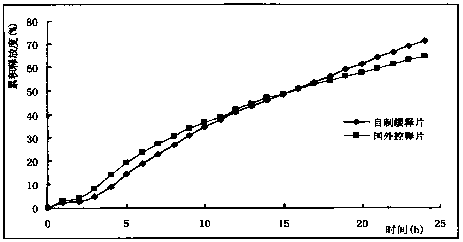
Nifedipine sustained release tablets and preparation process thereof
A preparation process, a technology for nifedipine, which is applied in the directions of pill delivery, cardiovascular system diseases, pharmaceutical formulations, etc., can solve the problems of short duration of action, accompanied by adverse reactions, complicated preparation process, etc., and achieves a slow dissolution rate, convenient for The effect of mass production and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The nifedipine sustained-release tablet of the present invention is prepared from the following raw materials and auxiliary materials in the weight ratio:
[0018] Nifedipine 20%
[0019] Chitosan 14%
[0020] Dextrin 60%
[0021] Ethylcellulose 4%
[0022] Sodium Lauryl Sulfate 1%
[0024] Wherein nifedipine is used as raw material, chitosan is used as sustained-release agent, dextrin is used as filler, ethyl cellulose is used as binder, sodium lauryl sulfate is used as solubilizer, and magnesium stearate is used as lubricant.
[0025] Preparation process: crush nifedipine and pass through a 200-mesh stainless steel sieve; mix nifedipine, chitosan, sodium lauryl sulfate and dextrin according to weight percentage; Add 80% ethanol to the cellulose to make soft material from ethyl cellulose with a weight concentration of 8%—80% ethanol solution; pass the soft material through a 20-mesh stainless steel sieve to make wet granules; put the...
Embodiment 2
[0027] The nifedipine sustained-release tablet of the present invention is prepared from the following raw materials and auxiliary materials in the weight ratio:
[0028] Nifedipine 20%
[0029] Chitosan 17%
[0030] Dextrin 57%
[0031] Ethylcellulose 4%
[0032] Sodium Lauryl Sulfate 1%
[0034] Among them, nifedipine is used as raw material, chitosan is used as slow-release agent, dextrin is used as filler, ethyl cellulose is used as binder, sodium lauryl sulfate is used as solubilizer, and magnesium stearate is used as lubricant.
[0035] Preparation process: crush nifedipine and pass through a 200-mesh stainless steel sieve; mix nifedipine, chitosan, sodium lauryl sulfate and dextrin according to weight percentage; add ethyl cellulose after the raw and auxiliary materials are evenly mixed - 80% ethanol solution to make soft material; pass the soft material through a 20-mesh stainless steel sieve to make wet granules; dry the wet granule...
Embodiment 3
[0037]The nifedipine sustained-release tablet of the present invention is prepared from the following raw materials and auxiliary materials in the weight ratio:
[0038] Nifedipine 20%
[0039] Chitosan 20%
[0040] Dextrin 54%
[0041] Ethylcellulose 4%
[0042] Sodium Lauryl Sulfate 1%
[0044] Wherein nifedipine is used as raw material, chitosan is used as sustained-release agent, dextrin is used as filler, ethyl cellulose is used as binder, sodium lauryl sulfate is used as solubilizer, and magnesium stearate is used as lubricant.
[0045] Preparation process: crush nifedipine and pass through a 200-mesh stainless steel sieve; mix nifedipine, chitosan, sodium lauryl sulfate and dextrin according to weight percentage; add ethyl cellulose after the raw and auxiliary materials are evenly mixed - 80% ethanol solution to make soft materials; pass the soft materials through a 20-mesh stainless steel sieve to make wet granules; dry the wet granu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com