Co-containing sandwich heteropolyacid as well as synthesis method and application thereof
A synthesis method and technology of heteropolyacids, applied in cobalt compounds, chemical instruments and methods, drug combinations, etc., can solve the problems that polyoxometalates have not yet been developed, and research has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
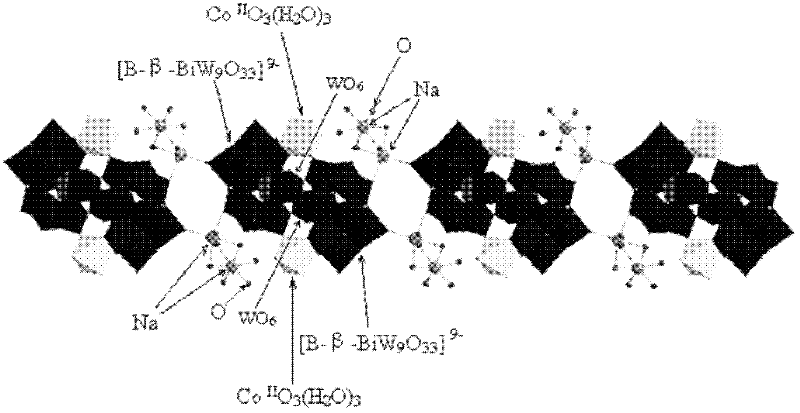
[0011] Specific embodiment one: the molecular formula of the cobalt-containing sandwich heteropolyacid of the present embodiment is H 6 [Bi 2 W 20 co 2 (OH 2 ) 6 o 70 Na 4 (OH 2 ) 14 ](C 3 h 4 N 2 ) 2 .
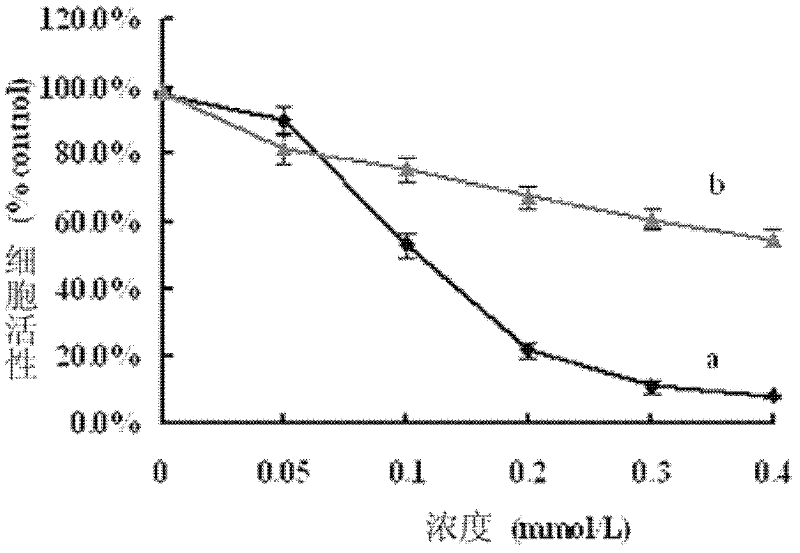
[0012] H in this embodiment 6 [Bi 2 W 20 co 2 (OH 2 ) 6 o 70 Na 4 (OH 2 ) 14 ](C 3 h 4 N 2 ) 2It is pink powder crystal. H in this embodiment 6 [Bi 2 W 20 co 2 (OH 2 ) 6 o 70 Na 4 (OH 2 ) 14 ](C 3 h 4 N 2 ) 2 The growth inhibitory effect of the compound on human colon cancer HT-29 tumor cells was detected by thiazolium blue (MTT) method, and its half inhibitory concentration IC 50 =0.11 mmol / L, 24h. Fluorescent staining methods such as Hoechst 33342 and AO / EB detect the cobalt-containing sandwich heteropolyacid H in the present embodiment. 6 [Bi 2 W 20 co 2 (OH 2 ) 6 o 70 Na 4 (OH 2 ) 14 ](C 3 h 4 N 2 ) 2 It has apoptosis-inducing effect on human colon tumor cell lines. Adopt the agarose gel electrophoresis (DNAladder) ...
specific Embodiment approach 2
[0013] Specific embodiment two: the synthetic method of the cobalt-containing sandwich heteropolyacid of the present embodiment is carried out according to the following steps: one, under magnetic stirring, the Na of 4mmol~5mmol 2 WO 4 Dissolve in 80mL-120mL deionized water, and heat to 80°C-120°C, then add hydrochloric acid with a concentration of 6mol / L dropwise to adjust the pH to 5.0-7.0 to obtain solution A; 2. Mix 0.3mmol-0.8mmol of Bi(NO 3 ) 3 Dissolved in 10 mL of hydrochloric acid with a concentration of 6 mol / L, Bi(NO 3 ) 3 solution, and Bi(NO 3 ) 3 Solution, 0.5mmol ~ 1mmol CoCl 2 Add 0.8mmol ~ 1.2mmol imidazole to the solution A obtained in step 1 and mix evenly, heat to 80°C ~ 120°C and keep it for 1h ~ 2h under stirring condition, then cool to room temperature, after filtering, the filtrate is still for 5 After ~10 days, crystals were precipitated to obtain a cobalt-containing sandwich heteropolyacid.
specific Embodiment approach 3
[0014] Specific embodiment three: the difference between this embodiment and specific embodiment two is that in step one, 4.2mmol~4.8mmol of Na 2 WO 4 Dissolve in 90mL~110mL deionized water, and heat to 90℃~110℃. Other steps and parameters are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com