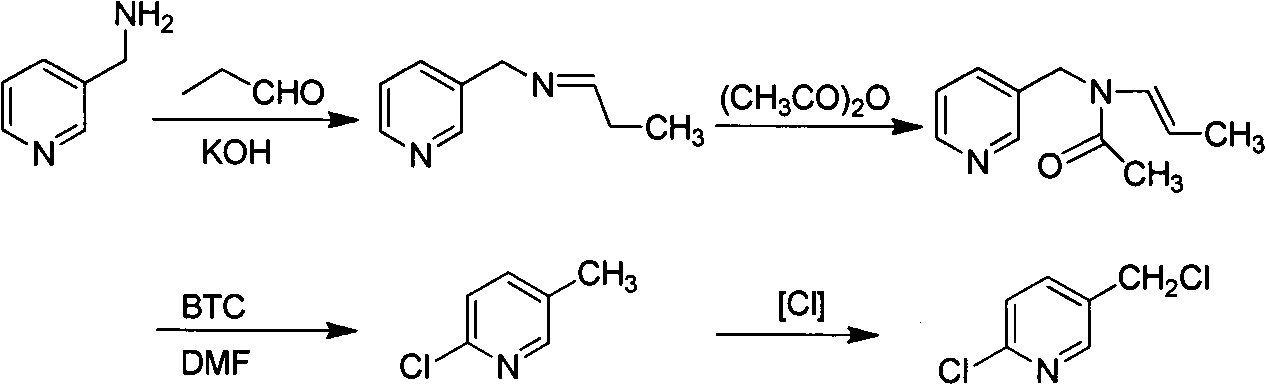
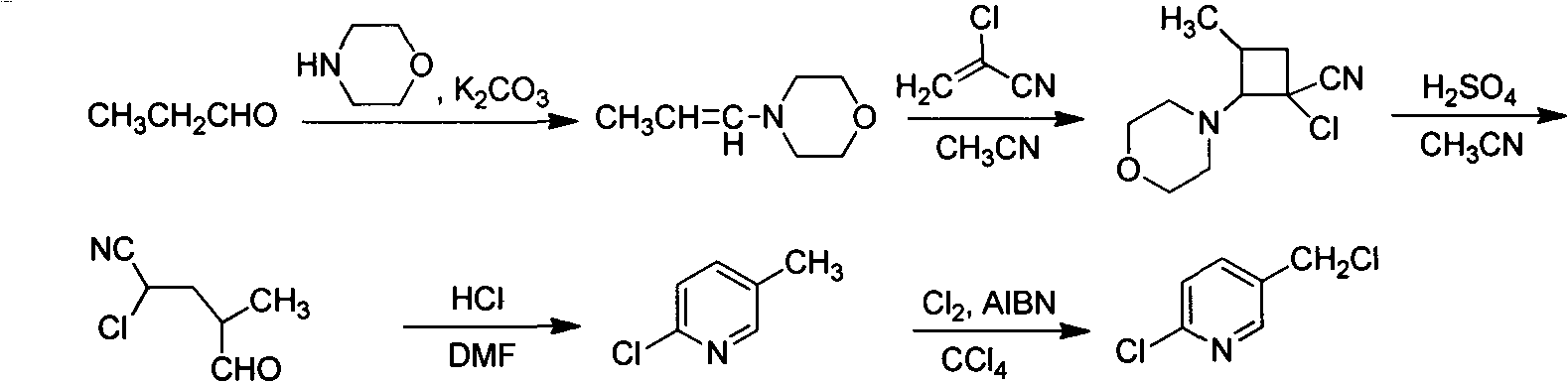
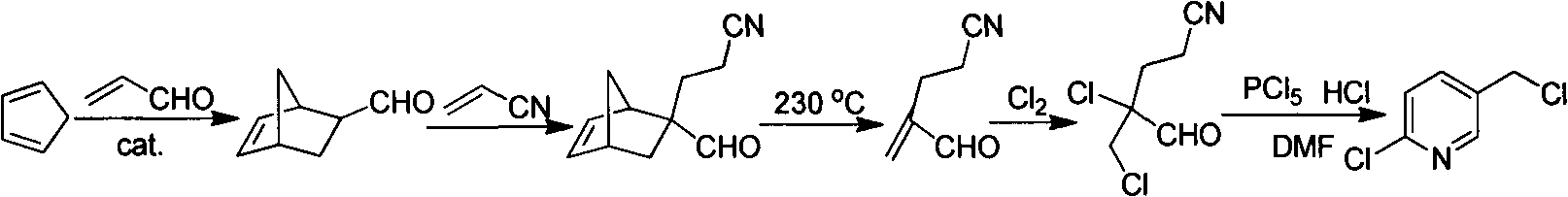
Improved 2-chlorine-5-chloromethyl pyridine synthesis process
A kind of chloromethylpyridine and synthesis technology technology, applied in the direction of organic chemistry, etc., can solve problems such as environmental impact, no recovery method, etc., to achieve the effect of overcoming a large amount of waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Chlorination addition reaction
[0027] 2-methenyl-4-cyanobutyraldehyde (109 g, 1.00 mol) and toluene (300 g) were successively put into a 1000 ml three-necked flask. Control the temperature at -5-2°C, and feed chlorine gas. When the content of 2-methenyl-4-cyanobutyraldehyde is less than 1% as detected by gas chromatography, stop the chlorine gas flow. Raise the temperature to 30°C, and remove excess chlorine under reduced pressure. When the material turns from dark yellow to light yellow and transparent, stop the gas removal to obtain a toluene solution of 2-chloro-2-chloromethyl-4-cyanobutyraldehyde.
Embodiment 2
[0028] Example 2 Cyclization reaction
[0029] DMF (21.9g, 0.3mol) was added to the reaction solution in Example 1, and the temperature was raised to 90°C to start dropwise addition of a toluene (300g) solution containing triphosgene (100g, 0.340mol). The dropping time was 2h, and the temperature was controlled at 90 ~100°C, keep warm for 3-6h after the dropwise addition, gas chromatography detects that the content of 2-chloro-2-chloromethyl-4-cyanobutyraldehyde is less than 1.0%, and the content of 2-chloro-5-chloromethylpyridine reaches 97.0% %, stop the reaction. At 55° C., triethylamine (212 g, 2.09 mol) was added dropwise, then vacuum filtered while hot, and the filter cake was rinsed with hot toluene (50 ml×2 times). The toluene layers were combined, the toluene was distilled off, and then distilled under reduced pressure to collect fractions at 110-115°C / 1600kPa to obtain refined 2-chloro-5-chloromethylpyridine (118g, 0.727mol) with a yield of 72.7%.
Embodiment 3
[0030] Example 3 Cyclization reaction
[0031] DMF (21.9g, 0.3mol) was added to the reaction solution in Example 1, and the temperature was raised to 85°C, and a toluene (300g) solution containing triphosgene (100g, 0.340mol) was added dropwise for 2 hours, and the temperature was controlled at 90°C. ~100°C, keep warm for 3-6 hours after the dropwise addition, the content of 2-chloro-2-chloromethyl-4-cyanobutyraldehyde is less than 1.0% and the content of 2-chloro-5-chloromethylpyridine reaches 97.0% as detected by GC , stop the reaction. At 45° C., tri-n-propylamine (299 g, 2.09 mol) was added dropwise, then vacuum filtered while hot, and the filter cake was rinsed with hot toluene (50 ml×2 times). The toluene layers were combined, the toluene was distilled off, and then distilled under reduced pressure to collect fractions at 110-115°C / 1600kPa to obtain refined 2-chloro-5-chloromethylpyridine (114g, 0.704mol) with a yield of 70.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com