Bishydroxyl sulfoacid betaine surface active agent and synthesis method thereof
A technology of surfactants and dihydroxysulfonic acid, applied in the direction of sulfonate preparation, chemical instruments and methods, and drilling compositions, etc., can solve the problem of gemini surfactants and dibetaine surfactants, which are rarely reported and other problems, to achieve the effect of simple synthesis method, superior surface activity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 DBA-12
[0034] (1) Synthesis of N-(sodium 2-hydroxypropanesulfonate)-N-dodecylamine
[0035] In a three-necked flask equipped with a stirrer placed in a constant temperature water bath, 4.63g (0.025mol) of dodecylamine, 5g (0.025mol) of 3-chloro-2-hydroxypropanesulfonate, and 100mL of 70% ethanol aqueous solution were sequentially added (as a solvent). Stir and reflux. Because dodecylamine itself is basic, and too strong basicity is unfavorable to the alkylation of amine, so 1g (20%wt) of Na was added after reflux for about 3h 2 CO 3 As an acid-binding agent, keep the pH of the system at 7-10. After refluxing for 10 h, the reaction ended (TLC monitored the end point of the reaction, and the developer was V (methanol): V (petroleum ether) = 3:10). The generated salt was filtered off while it was hot, and the filtrate was cooled to room temperature, and white crystals were precipitated. Suction filtration, wash the filter cake three ...
Embodiment 2
[0040] The preparation of embodiment 2 DBA-14
[0041] (1) Synthesis of N-(sodium 2-hydroxypropanesulfonate)-N-tetradecylamine
[0042] The reaction device is the same as in Example 1 (1), and 6.04g (0.025mol) tetradecylamine and 5g (0.025mol) 3-chloro-2-hydroxypropanesulfonate sodium are added successively in the reactor, and 100mL70% ethanol aqueous solution ( as a solvent). Stir and reflux. After reflux for about 3h, add 1.2g (20%wt) of Na 2 CO 3 As an acid-binding agent, keep the pH of the system at 7-10. After refluxing for another 12 hours, the reaction ended (TLC monitored the end point of the reaction, and the developer was V (methanol): V (petroleum ether) = 3:10). The generated salt was filtered off while it was hot, and the filtrate was cooled to room temperature, and white crystals were precipitated. Suction filtration, wash the filter cake three times with 150ml benzene to remove unreacted tetradecylamine, then recrystallize twice at room temperature with 10...
Embodiment 3
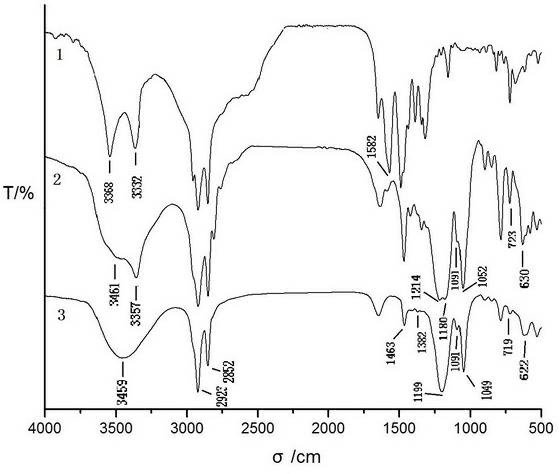
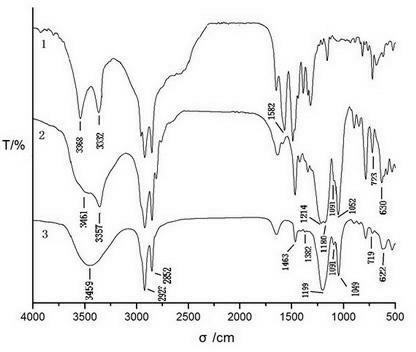
[0047] The infrared spectrogram of embodiment 3 raw material, intermediate product and target product
[0048] Raw material primary amine, the infrared spectrogram of the first-order intermediate product that obtains by embodiment example 1 and target product sees figure 1 . Among the figure, 1 is dodecylamine, 2 is N-(sodium 2-hydroxypropanesulfonate)-N-dodecylamine, and 3 is DBA-12. From the analysis of the spectrogram, it can be seen that:
[0049] In the IR spectrum of the raw material dodecylamine, there are N-H symmetric stretching (3332), asymmetric stretching (3368), and in-plane bending (1582) vibration peaks of primary amines; in the IR spectrum of the intermediate product, these primary amines The characteristic bands of all disappeared, secondary amine N-H stretching vibration (3357), O-H stretching vibration (3461), C-O stretching vibration (1052), S=O symmetrical stretching vibration (1091), asymmetric stretching vibration (1180), Characteristic bands of S-O s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com