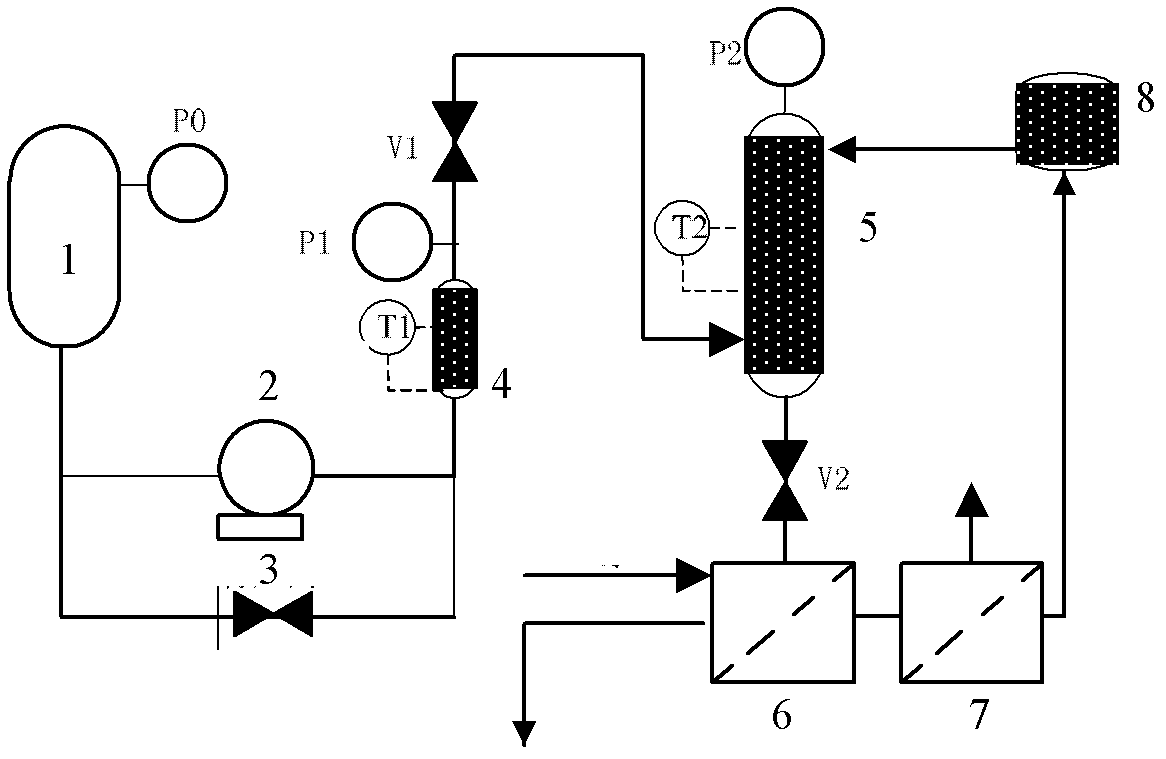
Method for preparing nanometer calcium carbonate
A technology of nano-calcium carbonate and nano-particles, applied in the direction of calcium carbonate/strontium/barium, nanotechnology, bulk chemical production, etc., can solve the problems of slow reaction and low efficiency, and achieve the effect of improving reaction rate and convenient selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (anhydrous process)
[0034] Raw materials include: CO 2 (Xiamen Tongan Air Separation Special Gas Plant, purity > 98%), tetraheptyl ammonium bromide [THepAm] [Br] (Shanghai Mairuier Chemical Technology Co., Ltd., purity > 99%), Ca(OH) 2 (Sinopharm Chemical Reagent Co., Ltd., analytically pure, 95%).
[0035] Dry Ca(OH) with a mass ratio of 1:1 2 Mix it with tetraheptylammonium bromide in a mixer, send the mixture into the autoclave, heat the autoclave to 50°C, open the carbon dioxide supply part, make the carbon dioxide pressure 15MPa, and pass it into the autoclave. The reaction was stirred for 5 min and 30 min. The reaction product is released from the high-pressure reaction kettle, washed with the same volume of water, the ionic liquid is washed into the water, and the water is evaporated to recover the ionic liquid. The washed slurry solid is directly heated and dried to obtain the product.
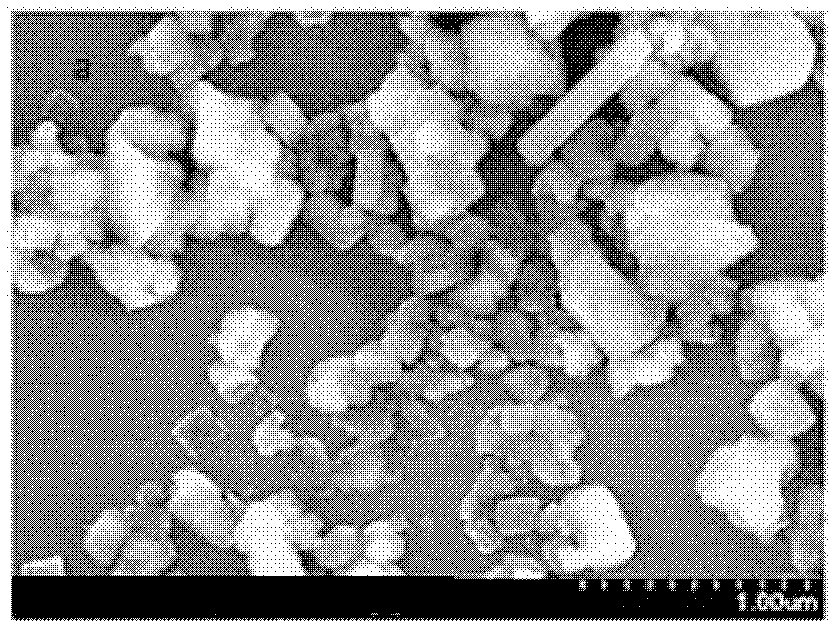
[0036] figure 2 The SEM image of the product reacted ...
Embodiment 2
[0037] Embodiment 2 (anhydrous process)
[0038] Raw materials include: CO 2 (Tongan Air Separation Special Gas Plant, Xiamen City, purity 98%), 1-butyl-3-methylimidazolium chloride [Bmim] [Cl] (Tixiai (Shanghai) Chemical Industry Development Co., Ltd., purity > 98% ). Ca(OH) 2 (Sinopharm Chemical Reagent Co., Ltd., analytically pure, 95%).
[0039] Dry Ca(OH) with a mass ratio of 1:1 2 Mix with 1-butyl-3-methylimidazolium chloride in a mixer, send the mixture into the autoclave, heat the autoclave to 50°C, open the carbon dioxide supply part, make the carbon dioxide pressure 15MPa, and pass it into the autoclave. The reaction was stirred for 5 minutes. The reaction product is released from the high-pressure reaction kettle, washed with the same volume of water, the ionic liquid is washed into the water, and the water is evaporated to recover the ionic liquid. The washed slurry solid is directly heated and dried to obtain the product.
[0040] Figure 4 The SEM picture...
Embodiment 3
[0041] Embodiment 3 (anhydrous process)
[0042] Raw materials include: CO 2 (Xiamen Tongan Air Separation Special Gas Plant, purity 98%), 1-butyl-3-methylimidazole borate [Bmim] [BF 4 ] (Shanghai Chengjie Chemical Co., Ltd., purity > 99%). Ca(OH) 2 (Sinopharm Chemical Reagent Co., Ltd., analytically pure, 95%).
[0043] Dry Ca(OH) with a mass ratio of 1:1 2 Mix with 1-butyl-3-methylimidazolium borate in a mixer, send the mixture into the autoclave, heat the autoclave to 50°C, open the carbon dioxide supply part, make the carbon dioxide pressure 15MPa, and pass it into the autoclave . The reaction was stirred for 5 minutes. The reaction product is released from the high-pressure reaction kettle, washed with the same volume of water, the ionic liquid is washed into the water, and the water is evaporated to recover the ionic liquid. The washed slurry solid is directly heated and dried to obtain the product.
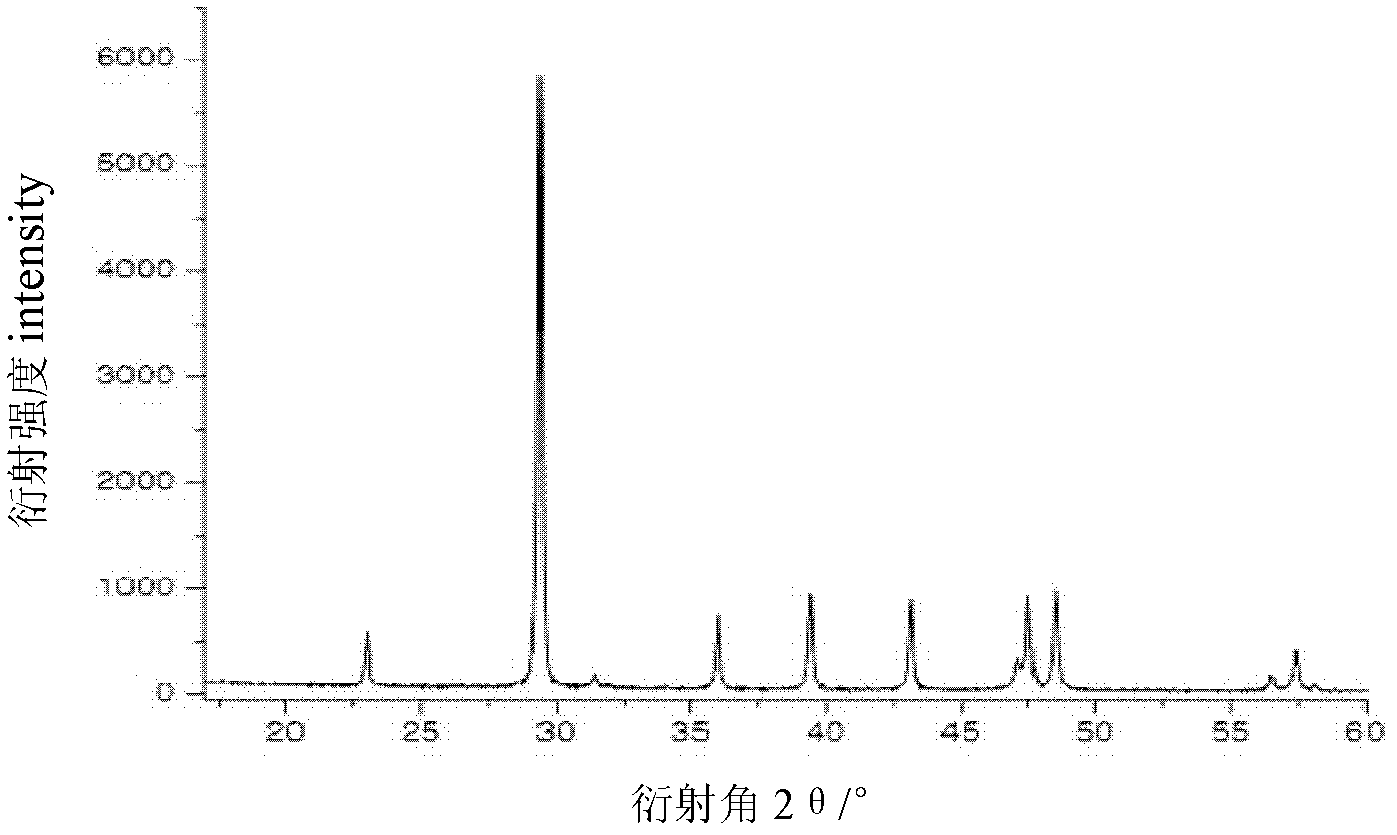
[0044] Calcium carbonate particles are about 100-500nm analyze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com