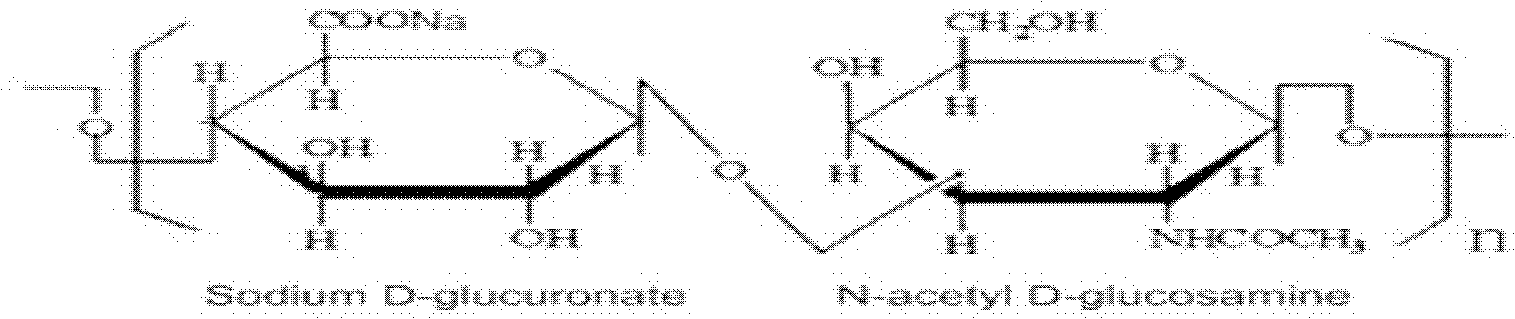
Dissolving method of sodium hyaluronate for solution preparation
A technology for sodium hyaluronate and liquid dispensing, which is applied in the directions of dissolution, dissolution, chemical instruments and methods, etc., can solve the problems of long dissolution time, difficulty in cleaning and sterilization, and reduce the coefficient of liquid filling, so as to expand the surface contact area and improve the non-toxicity. The level of bacteria protection and the effect of improving aseptic protection and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
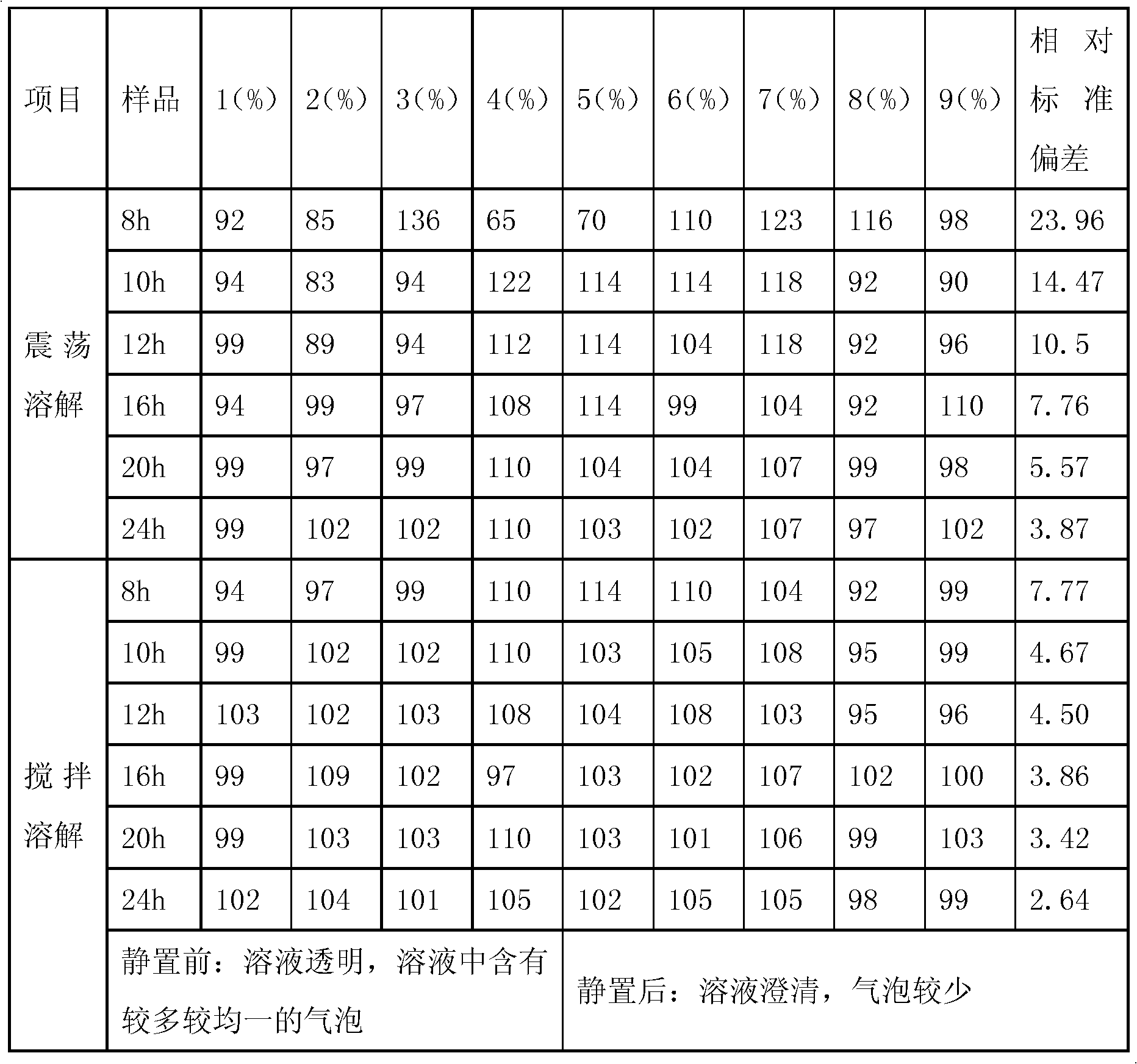
[0036] Stir and dissolve: when preparing sodium hyaluronate, add 15kg of PBS solution, set the frequency to 40Hz to start stirring, control the stirring speed at 120 rpm, add powder, granule or fibrous sodium hyaluronate to dry and pure amount After sodium hyaluronate is added, close the feeding port, fill the liquid mixing tank with inert gas (nitrogen, carbon dioxide, etc.) to control the pressure within the range of 0-0.1MPa. Continue to stir for 40-60 minutes, stop stirring, start stirring and dissolving for 1 hour after swelling for 1 hour, and then stir again for 1 hour every 1 hour. After adding sodium hyaluronate, stir and dissolve for 12 hours in total, stop stirring and control the inert gas (nitrogen , carbon dioxide gas, etc.) under the protection of 0.3MPa positive pressure for 8 hours.
[0037] Vibration and dissolution: In the existing sodium hyaluronate solution stage, add 15kg of PBS solution, set the vibration frequency to 40Hz and start stirring, add the sod...
Embodiment 2
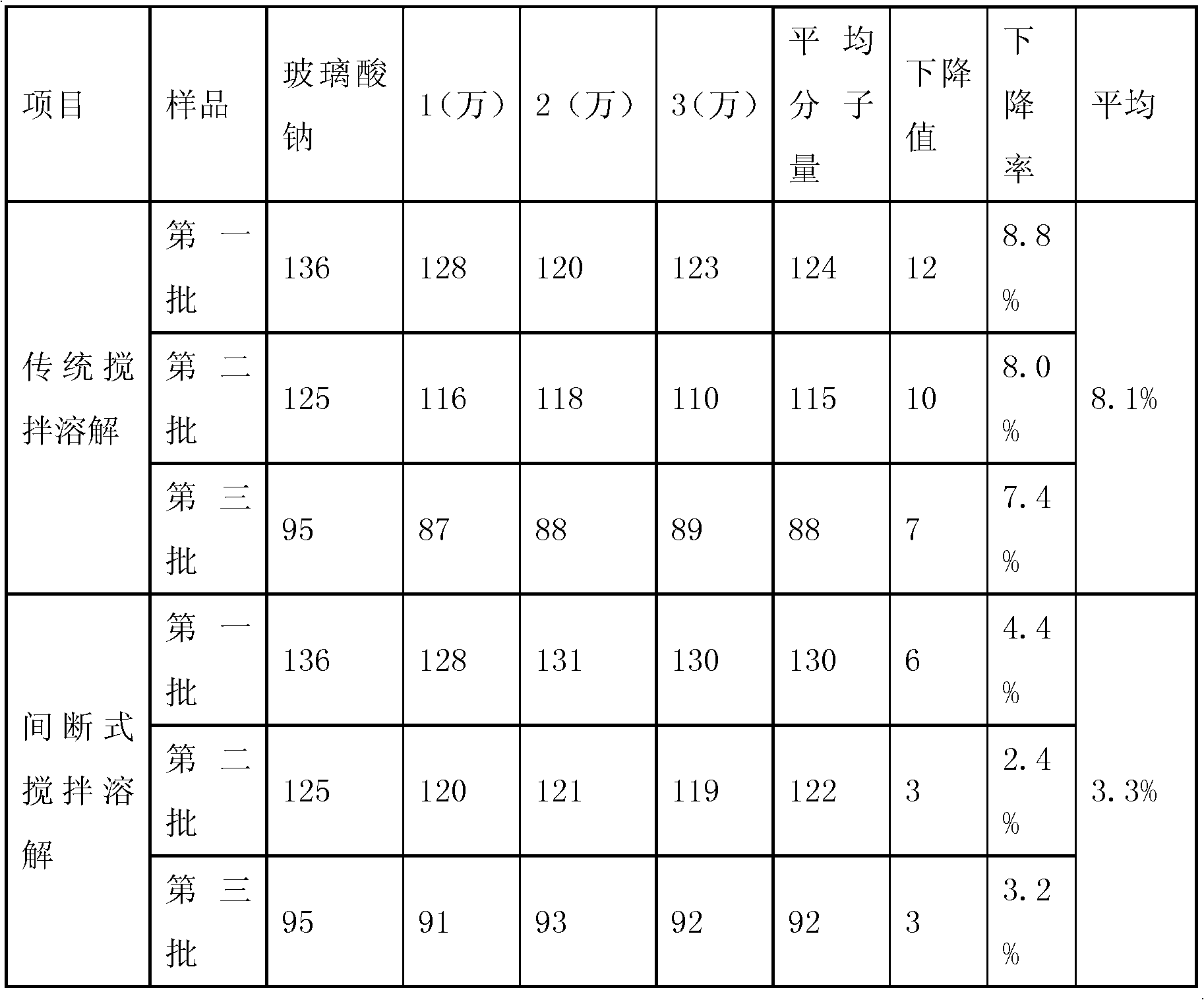
[0045] Traditional stirring and dissolving: In the existing sodium hyaluronate solution stage, add 15kg of PBS solution, set the frequency to 50Hz to start stirring, add the sodium hyaluronate prepared by the existing extraction method to a dry and pure amount of 153g, and stir while adding , after adding sodium hyaluronate, close the feeding port, continue to stir and dissolve for 12 hours, and stop stirring. According to this method, 3 batches were produced.
[0046] Intermittent stirring and dissolving: In the existing sodium hyaluronate solution stage, add 15kg of PBS solution, set the frequency to 40Hz to start stirring, control the stirring speed at 120 rpm, add the same batch of sodium hyaluronate prepared by the existing extraction method and fold dry The amount after conversion is 153g, stir while adding, after the sodium hyaluronate is added, close the feeding port, fill the inert gas (nitrogen, carbon dioxide gas, etc.) that is filtered through a 0.2um sterilizing f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com