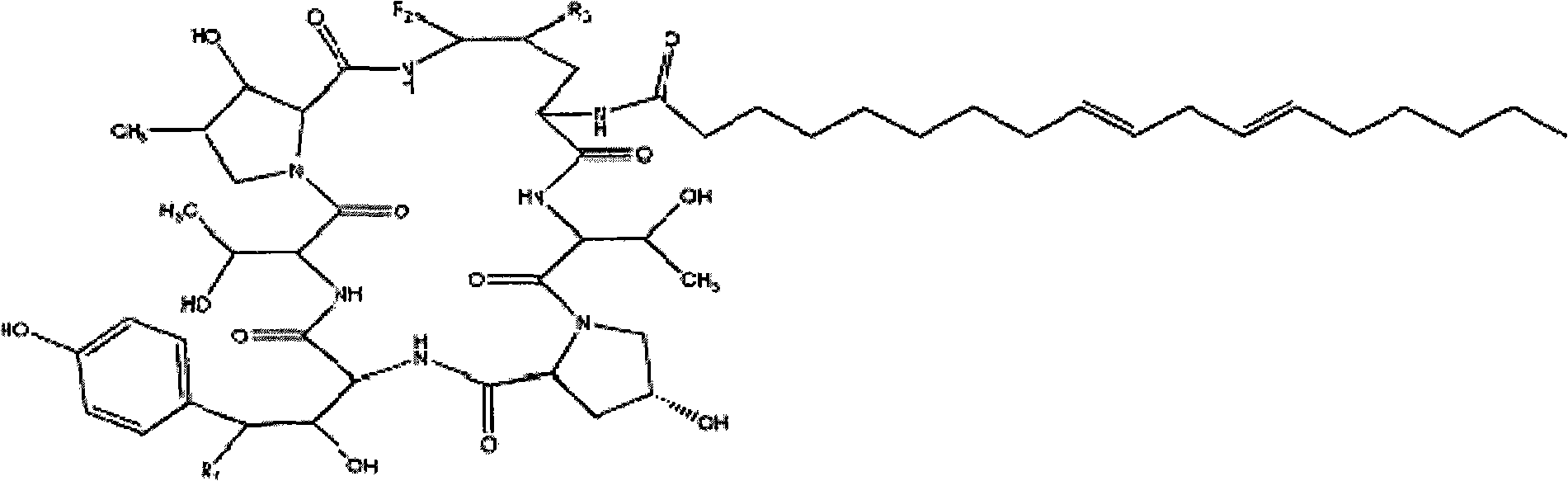
Method for obtaining high purity echinocandin D
A high-purity echinocandin technology, applied in the field of obtaining high-purity echinocandin D, can solve the problems of high cost, difficulty in large-scale application, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
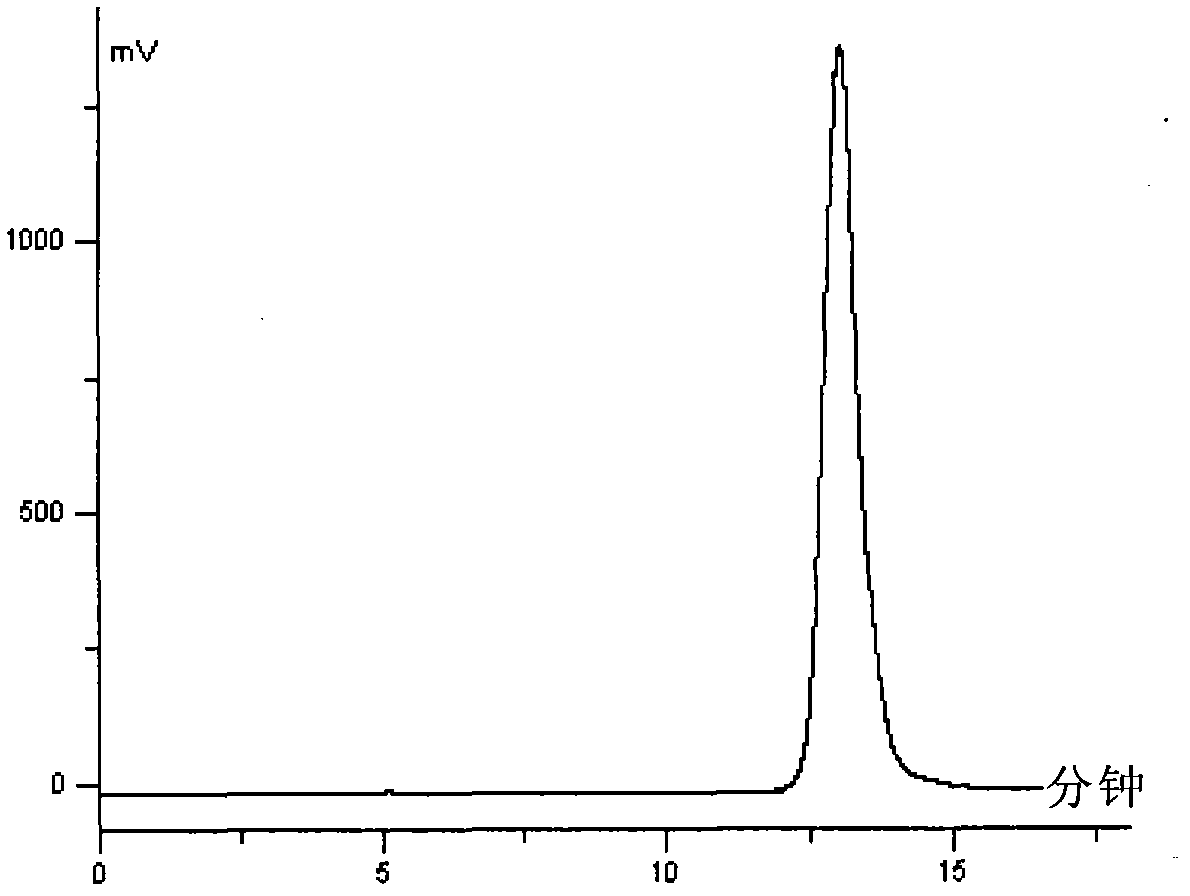
[0069] Load 100g NM-PS100 (4×20cm) reversed-phase column, dissolve 0.5g sample containing 80% echinocandin D in 60% methanol aqueous solution, pass through the column, echinocandin D is completely adsorbed on the column, The column was eluted with 85% methanol aqueous solution, and the higher purity fractions in the eluate were collected by distribution. The HPLC purity was 95.5%, the yield was 98%, and it was white. See attached figure 1 .
Embodiment 2
[0070] Example 2 (Influence of methanol concentration used for sample loading on purification)
[0071] Load 100g of NM-PS100 (4×20cm) reversed-phase column, dissolve 0.5g of sample containing 80% echinocandin D in 70% methanol aqueous solution, pass through the column, echinocandin D is completely adsorbed on the column, The column was eluted with 85% methanol aqueous solution, and the higher-purity fraction in the eluate was distributed and collected. The detected purity was 92%, the yield was 98%, and it was pale yellow.
Embodiment 3
[0072] Example 3 (Influence of methanol concentration used for sample loading on purification)
[0073] Load 100g NM-PS100 (4×20cm) reversed-phase column, dissolve 0.5g sample containing 80% echinocandin D in 50% methanol aqueous solution, pass through the column, echinocandin D is completely adsorbed on the column, The column was eluted with 85% methanol aqueous solution, and the higher purity fractions in the eluate were distributed and collected. The detected purity was 95%, the yield was 98%, and it was white.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com