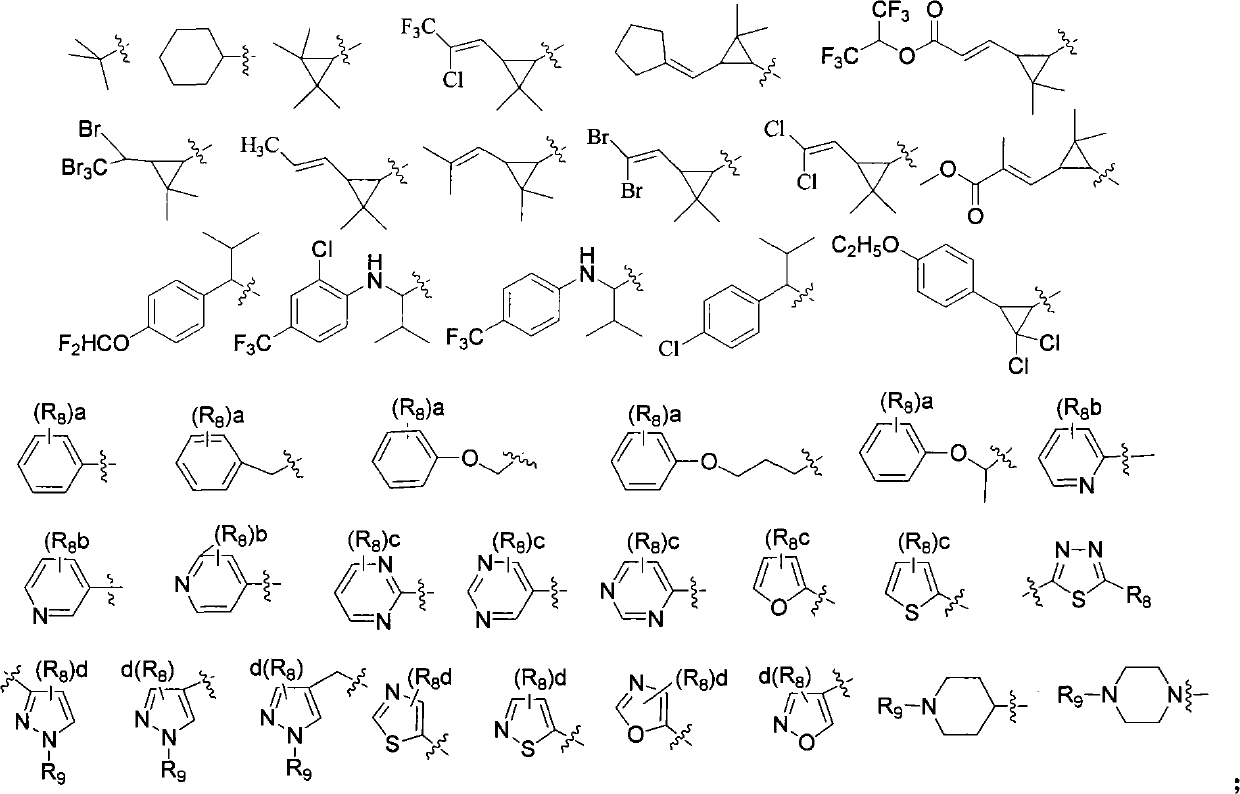
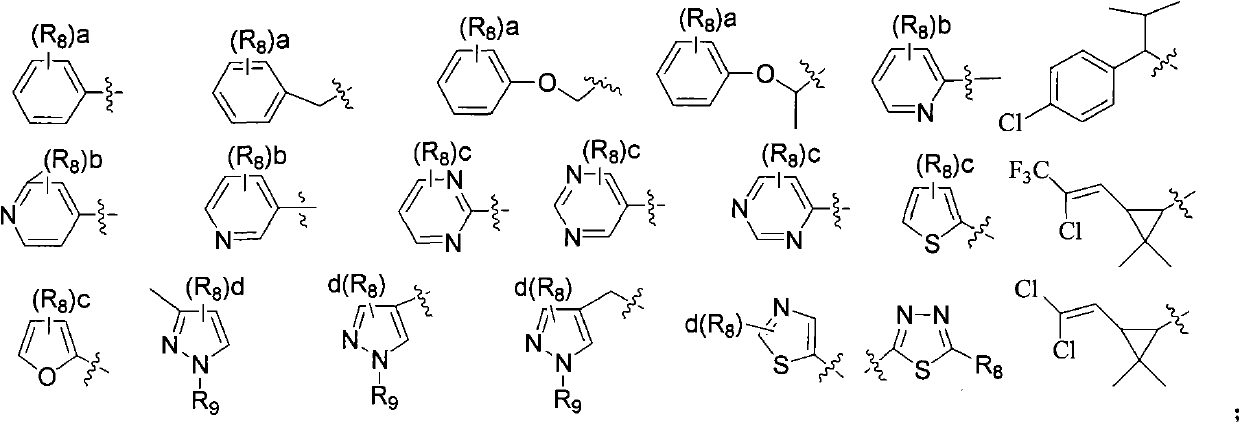
Acyl benzylamine compound and use thereof
A technology of acylbenzylamines and compounds, applied in the preparation and application of organic compounds, preparation of carboxylic acid amides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Embodiment 1: the preparation of intermediate II-1
[0186] 1).
[0187]
[0188] Add 19.80 g (0.067 mol) of solid phosgene in small portions to a solution containing 13.70 g (0.1 mol) of anthranilic acid in 150 ml of tetrahydrofuran under stirring at room temperature, and complete the addition in about 1.5 hours. Continue stirring at room temperature for 2-3 hours, TLC After monitoring the reaction, the solvent was evaporated under reduced pressure, the residue was added to 80ml of water, stirred until the excess phosgene was completely decomposed, filtered and washed with 50ml of water and petroleum ether in turn to obtain 14.00g of white solid, yield 86.0%, melting point 239- 240°C.
[0189] 2).
[0190]
[0191] Slowly add 40% methylamine aqueous solution dropwise to 100ml acetonitrile solution containing 16.30g (0.1mol) benzoxazinone under stirring at room temperature, until the solution becomes clear, add about 80ml methylamine aqueous solution dropwise, d...
Embodiment 2
[0201] Embodiment 2: the preparation of compound 23
[0202]
[0203] Add 0.313g (0.001mol) of benzylamine (II-1) and 0.12g (0.0012mol) of triethylamine into 20ml of dichloromethane, and add dropwise 0.17g (0.0011mol) of (III-1) in 10ml of dichloromethane under stirring at room temperature. Chloromethane solution, after dropping, continue stirring at room temperature for 1 hour, TLC monitors the reaction to the end point, pours the reaction mixture into 30ml of water, shakes and separates the organic layer, and the organic phase is sequentially washed with 10% dilute hydrochloric acid, saturated aqueous sodium bicarbonate solution, and saturated salt After washing with 20ml of water, the product was dried and precipitated to obtain the product, which was washed with ethyl acetate to obtain a white solid, and 0.38g of pure product was obtained by column chromatography, with a yield of 87.5% and a melting point of 168-169°C.
Embodiment 3
[0204] Embodiment 3: the preparation of compound 62
[0205]
[0206] Add 0.313g (0.001mol) of benzylamine (II-1) and 0.12g (0.0012mol) of triethylamine into 20ml of dichloromethane, and add dropwise 0.22g (0.0011mol) of (III-2) in 10ml of dichloromethane while stirring at room temperature. Chloromethane solution, after dropping, continue stirring at room temperature for 1 hour, TLC monitors the reaction to the end point, pours the reaction mixture into 30ml of water, shakes and separates the organic layer, and the organic phase is sequentially washed with 10% dilute hydrochloric acid, saturated aqueous sodium bicarbonate solution, and saturated salt The product was washed with 20 ml of water, dried and precipitated to obtain the product, washed with ethyl acetate to obtain a white solid, and 0.39 g of pure product was obtained by column chromatography, with a yield of 82.0% and a melting point of 154-156°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com