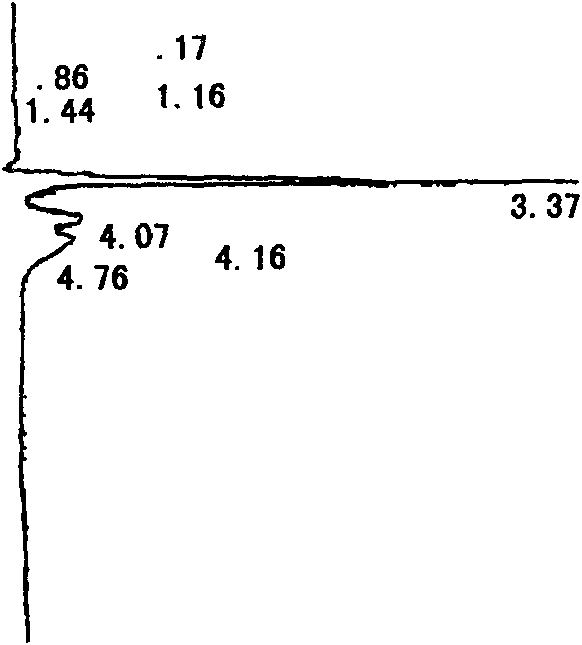
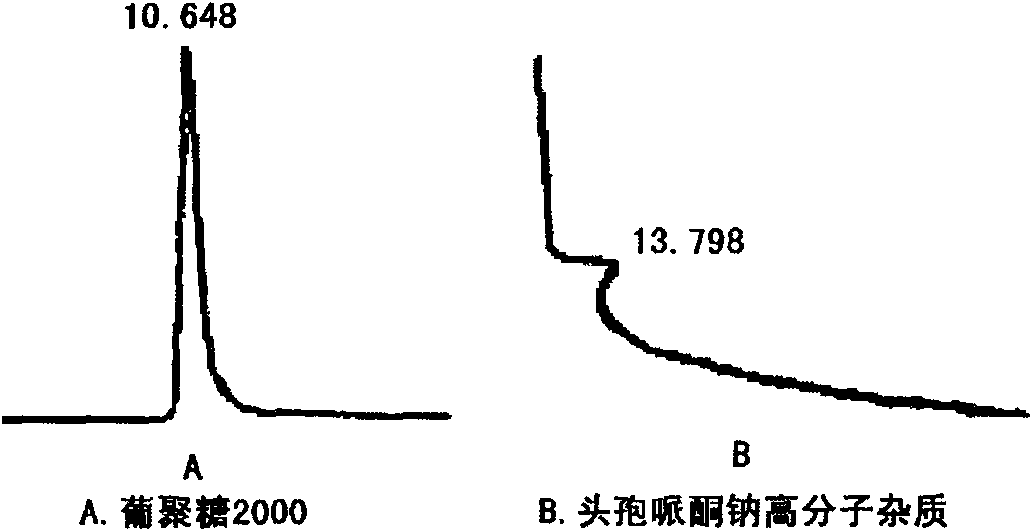
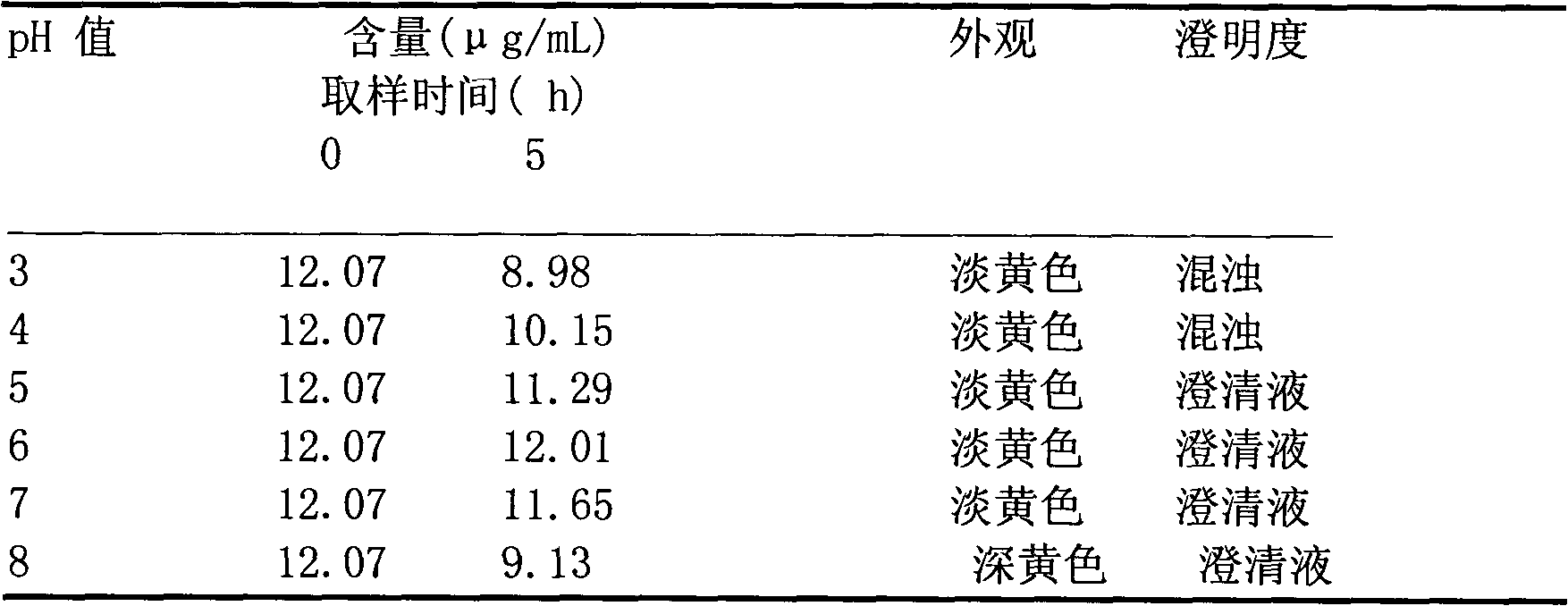
Medicinal composition consisting of ceftriaxone sodium and sulbactam sodium and preparation method thereof
A technology of ceftriaxone sodium and sulbactam sodium, which is applied in medical formulations, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., and can solve problems affecting the use of ceftriaxone sodium and sulbactam sodium , to achieve stable content, eliminate hidden dangers of medical accidents, and achieve remarkable clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]Under sterile and light-proof conditions, under sterile nitrogen (or other sterile inert gas) airflow, add 100g of ceftriaxone sodium (converted into anhydrous ceftriaxone sodium 100g), 50g of sulbactam sodium and 25g of Put mannitol into 90ml of 70% ethanol solution in a non-metallic container that has been cooled to below 10°C after pretreatment, stir to make it dissolve completely, drop in 0.1mol / L sodium bicarbonate aqueous solution pH value adjuster, adjust the pH value of the solution When the pH value reaches 6.5, filter aseptically, put it into a lyophilizer filled with inert gas for pre-freezing (-48~-20°C), decompress, sublimate, and dry to make a lyophilized eutectic powder raw material drug, and then Bacteria is packed into 91 bottles of powder injections to obtain 150 grams of eutectic bulk drug of the pharmaceutical composition of the present invention.
Embodiment 2
[0027] Under sterile and light-proof conditions, under sterile nitrogen flow, add 100g of ceftriaxone sodium (calculated as 100g of anhydrous ceftriaxone sodium), 45g of sulbactam sodium and 20g of mannitol into the pretreated It has been cooled to 80ml of 70% ethanol solution in a non-metallic container below 10°C, stir to dissolve completely, drop in 0.1mol / L sodium bicarbonate aqueous solution pH adjuster, adjust the pH of the solution to 6.3, and filter aseptically , put into a lyophilizer full of inert gas to pre-freeze (-48~-20 ℃), decompress, sublime, dry, and make lyophilized eutectic powder, which obtains the lyophilized powder of the pharmaceutical composition of the present invention. Preparation 94 bottles.
Embodiment 3
[0029] Under sterile and light-proof conditions, under sterile nitrogen flow, add 100g of ceftriaxone sodium (calculated as 100g of anhydrous ceftriaxone sodium), 50g of sulbactam sodium and 20g of lactose into the pretreated Cool to 90ml of 70% ethanol solution in a non-metallic container below 10°C, stir to make it dissolve completely, add dropwise 0.1mol / L sodium bicarbonate aqueous solution pH value regulator, adjust the pH value of the solution to 6.0, sterile filter, Put it into a lyophilizer filled with inert gas to pre-freeze (-48~-20°C), decompress, sublime, and dry to make lyophilized co-crystal powder, and then obtain the lyophilized preparation of the pharmaceutical composition of the present invention 93 bottles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com