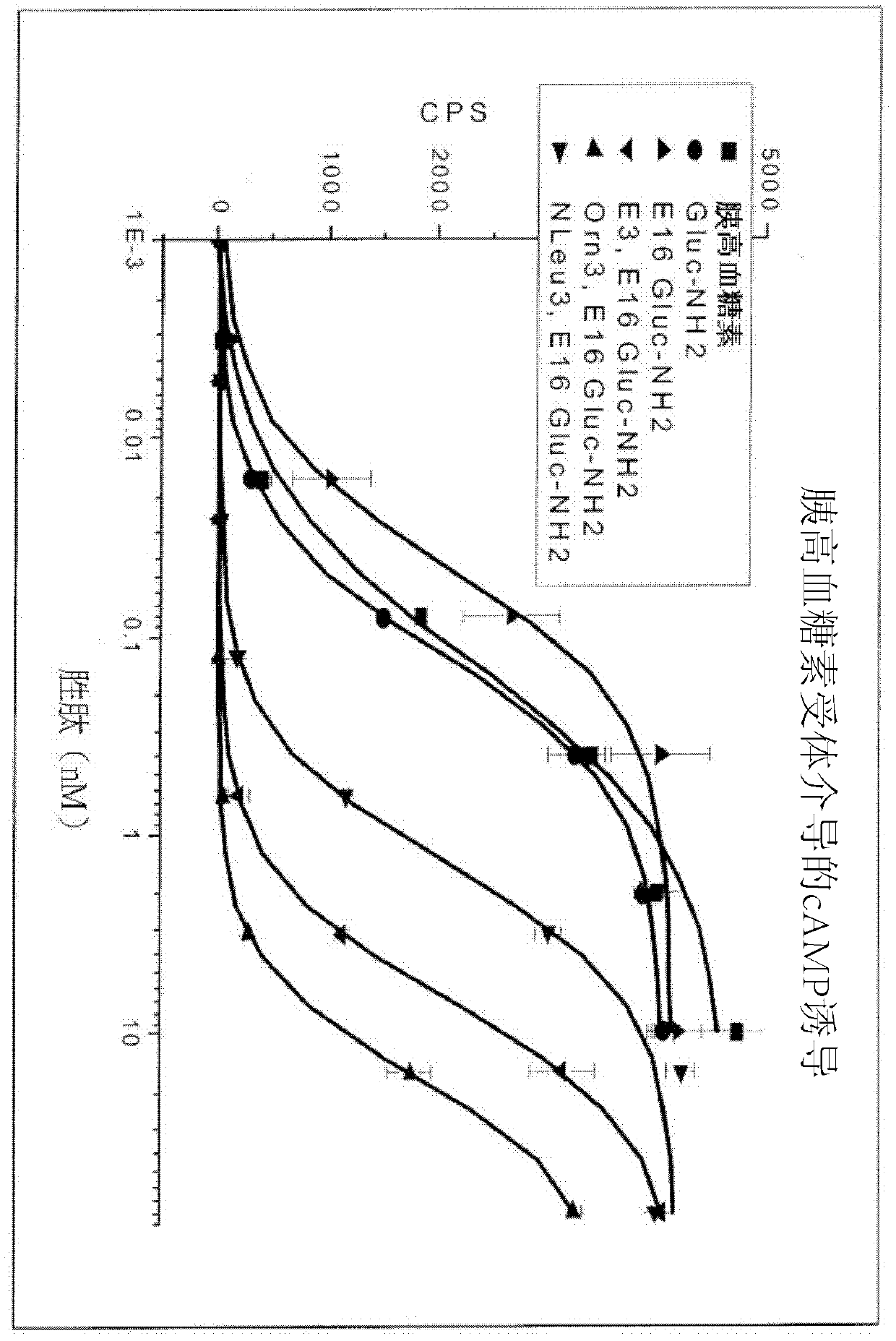
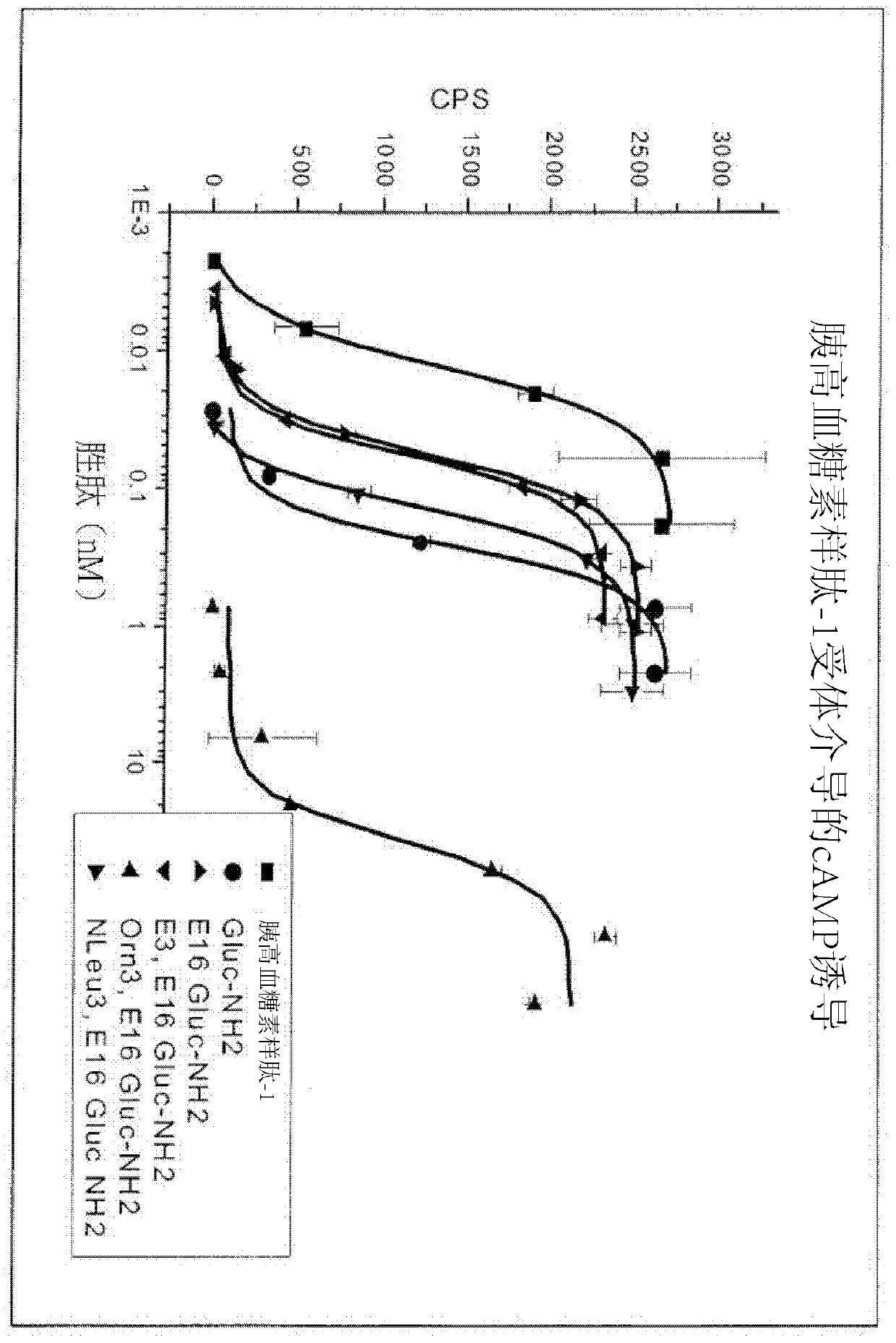
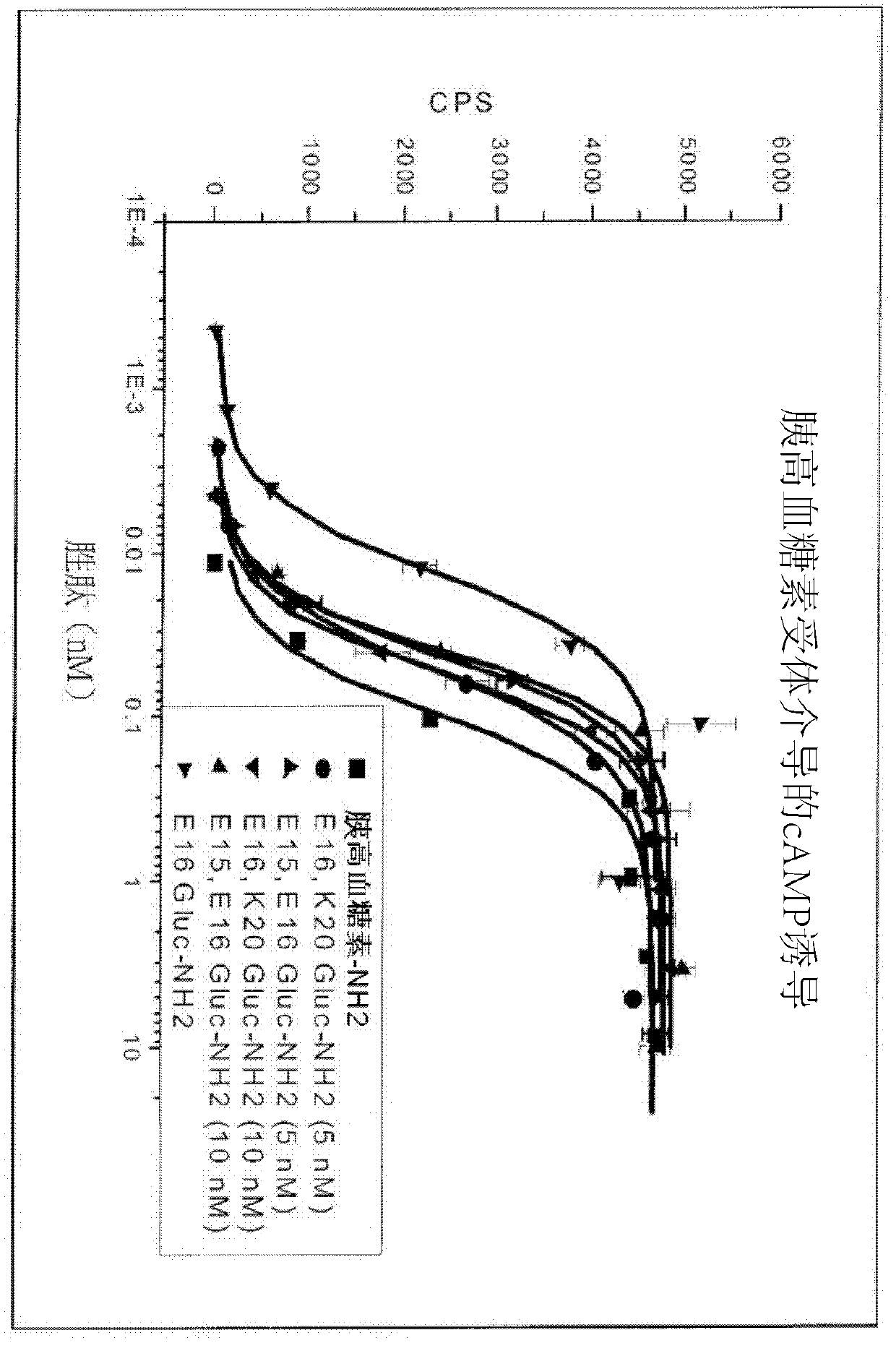
Gip receptor-active glucagon compounds
A glucagon and peptide-winning technology, applied in the direction of glucagon, hormone peptides, specific peptides, etc., can solve troublesome and dangerous problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0423] General synthetic experiment guidelines:
[0424] Glucagon analogs were synthesized on a modified Applied Biosystem Model 430A peptide synthesizer using HBTU-activated "Fast Boc" single coupler starting from 0.2 millimolar Boc Thr(OBzl)Pam resin. Boc-amino acids and HBTU were purchased from Midwest Biotech (Fishers, IN). The side chain protecting groups used are: arginine (Tos), asparagine (Xan), aspartic acid (OcHex), cysteine (pMeBzl), histidine (Bom), lysine acid (2Cl-Z), serine (OBzl), threonine (OBzl), tyrosine (2Br-Z), and tryptophan (CHO). The side chain protecting group on the histidine at the amino acid terminal is Boc.
[0425] Each completed peptidyl resin was treated with a 20% solution of piperidine in dimethylformamide (hereinafter referred to as DMF) to remove the formyl group from tryptophan. Liquid hydrogen fluoride (hydrofluoric acid, hereinafter referred to as HF) splitting is carried out in the presence of p-cresol and dimethyl sulfide. Dissoci...
Embodiment 1
[0430] Synthesis of Glucagon Cys17(1-29) and Similar Monocysteine Analogs Using FastBoc HBTU-activated single coupler, 0.2 millimolar Boc Thr(OBzl)Pam resin ( SynChem Inc) and the following sequences were imported and performed on a modified Applied Biosystems 430A peptide synthesizer.
[0431] HSQGTFTSDYSKYLDSCRAQDFVQWLMNT (SEQ ID NO: 35)
[0432] The following side chain protecting groups were used: arginine (Tos), aspartic acid (OcHex), asparagine (Xan), cysteine (pMeBzl), glutamic acid (OcHex), histidine (Boc), lysine (2Cl-Z), serine (Bzl), threonine (Bzl), tryptophan (CHO) and tyrosine (2Br-Z). The completed peptidyl resin was treated with 20% piperidine / dimethylformamide to remove the formyl protection of tryptophan, then transferred to an HF reaction vessel and dried in vacuo. Add 1.0 ml p-cresol and 0.5 ml dimethyl sulfide along with a magnetic stir bar. The vessel was connected to a HF apparatus (Pennisula Labs), cooled in a dry ice / methanol bath, evacuated, an...
Embodiment 2
[0435] Synthesis of Glucagon-Cex and Other Carboxy-Terminally Extended Analogues
[0436] 285 mg (0.2 mmol) of methoxybenzhydrylamine resin (Midwest Biotech) was placed in a 60 ml reaction vessel, and FastBoc HBTU-activated single coupler was used in the modified Applied Biosystems 430A peptide Enter and execute the following sequence on the synthesizer.
[0437] HSQGTFTSDYSKYLDSRRAQDFVQWLMNTGPSSGAPPPS (SEQ ID NO: 36)
[0438] The following side chain protecting groups were used: arginine (Tos), aspartic acid (OcHex), asparagine (Xan), cysteine (pMeBzl), glutamic acid (OcHex), histidine (Boc), lysine (2Cl-Z), serine (Bzl), threonine (Bzl), tryptophan (CHO) and tyrosine (2Br-Z). The completed peptidyl resin was treated with 20% piperidine / dimethylformamide to remove the formyl protection of tryptophan, then transferred to an HF reaction vessel and dried in vacuo. Add 1.0 ml p-cresol and 0.5 ml dimethyl sulfide along with a magnetic stir bar. The vessel was connected to a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com