Separation and purification method of c-di-GMP (cyclic diguanylate)
A c-di-gmp, separation and purification technology, applied in the field of separation and purification of cyclic guanosine diphosphate, can solve the problems of reducing the service life of the chromatographic column, hazards to the operator and the environment, and complicated separation and purification process, so as to reduce the separation and purification The effect of time, production cost reduction, and simplification of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1c
[0027] The preparation (enzymatic reaction method) of the crude product solution of embodiment 1c-di-GMP
[0028] GTP (purchased from Sigma) and the enzyme VCA0956 with c-di-GMP synthesis activity were added to the reaction buffer (75mM Tris-HCl pH 8.0, 250mM NaCl, 25mM KCl, 10mM MgCl 2 ), react overnight at room temperature to obtain a crude product solution containing c-di-GMP.
[0029] Among them: For the detailed methods and steps of the expression and purification of the above-mentioned VCA0956 and the preparation of the c-di-GMP crude product solution, please refer to the literature: Rita Tamayo, Anna D. Tischler, and Andrew Camilli. The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J. Biol. Chem., 2005, 280, 33324-33330.
Embodiment 2
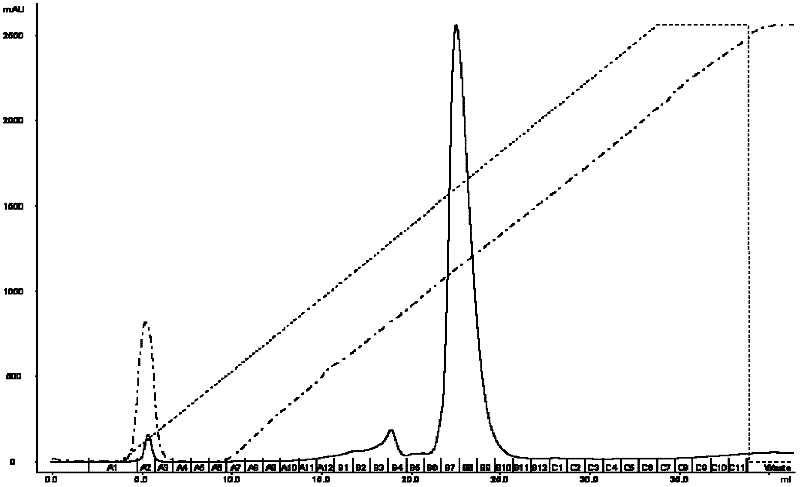
[0031] (1) Take 4 mL of the crude product solution containing c-di-GMP prepared in Example 1, and load it on the ion-exchange chromatographic column GE AKTA FPLC equipped with Mini Q 4.6 / 50PE at a flow rate of 1 mL / min;
[0032] (2) After loading the sample, use 25mM Tris-HCl (pH8.0) (A) and 25mM Tris-HCl (pH8.0) + 1M NaCl (B) gradient elution at a flow rate of 1.5mL / min, gradient elution The stripping is carried out at 0→20 times the column volume, the concentration value of eluent A is carried out at 100%→0, and the concentration value of eluent B is carried out at 0→100%;
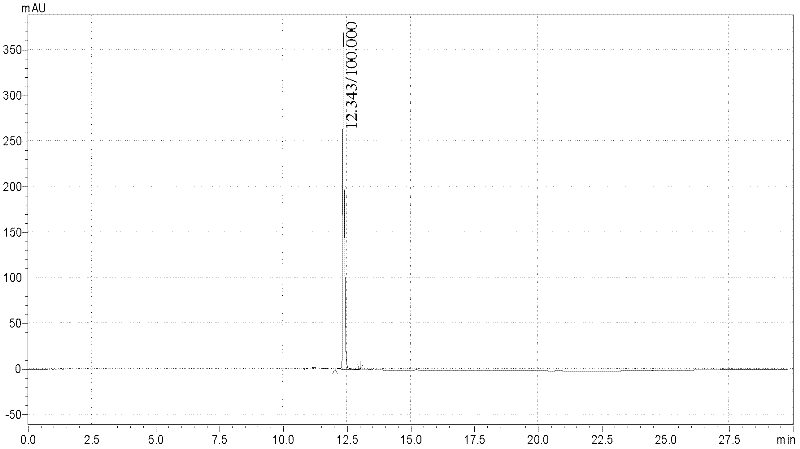
[0033](3) Use a UV detector to detect at 254nm; the peak appears when the conductivity of the eluent is 35.97mS / cm, collect the eluate at the position of the peak, and confirm its structure with mass spectrometry [(ESI+): m / e(relative intensity ): [M+1] + 691.2, [M+2]2+346.2], this part of the eluate was lyophilized to obtain solid c-di-GMP (results such as figure 1 shown);
[0034] (4) The product of...
Embodiment 3
[0036] (1) Get 30 mL of the crude product solution containing c-di-GMP prepared in Example 1, and load it on a GE AKTA FPLC equipped with a Source Q HP 10 / 10 ion-exchange chromatographic column at a flow rate of 3 mL / min;
[0037] (2) After loading the sample, use 25mM Tris-HCl (pH8.0) (A) and 25mM Tris-HCl (pH8.0) + 1M NaCl (B) gradient elution at a flow rate of 3mL / min, gradient elution Carry out at 0→20 times the column volume, the concentration value of eluent A is carried out at 100%→0, and the concentration value of eluent B is carried out at 0→100%;
[0038] (3) Detect at 254nm with an ultraviolet detector; when the eluent conductance is 36.03mS / cm, the peak is collected, and the eluent at the peak position is collected, and its structure is confirmed by mass spectrometry, and this part of the eluent is freeze-dried to obtain final product Solid c-di-GMP;
[0039] (4) The product of step (3) is detected by reverse-phase HPLC, and the high-performance liquid chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com