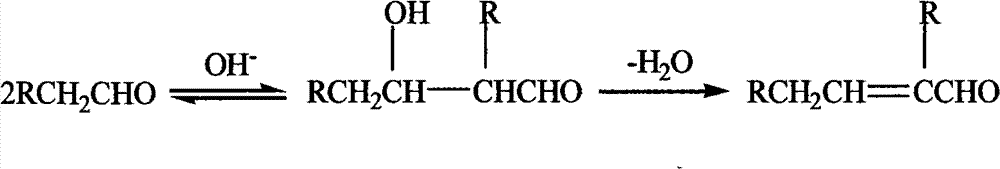
Preparation method of unsaturated aldehyde by aldehyde condensation
A technology of unsaturated and aldol condensation reaction, applied in the preparation of unsaturated aldehydes, the field of preparing unsaturated aldehydes by aldehyde condensation, can solve the problems of reducing yield, high heat, consumption, etc., to reduce the possibility and reduce the burden Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
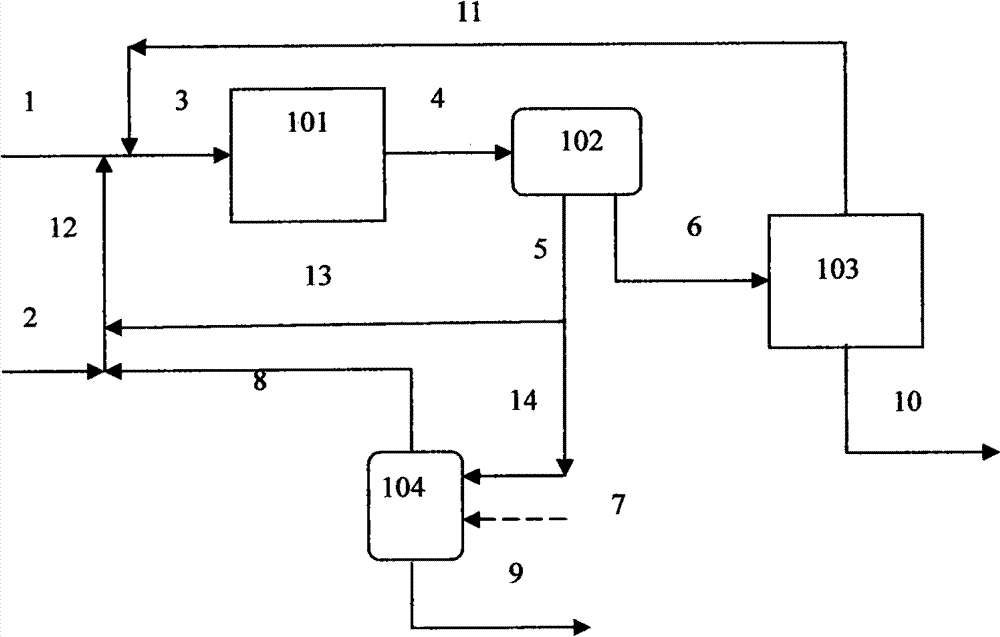
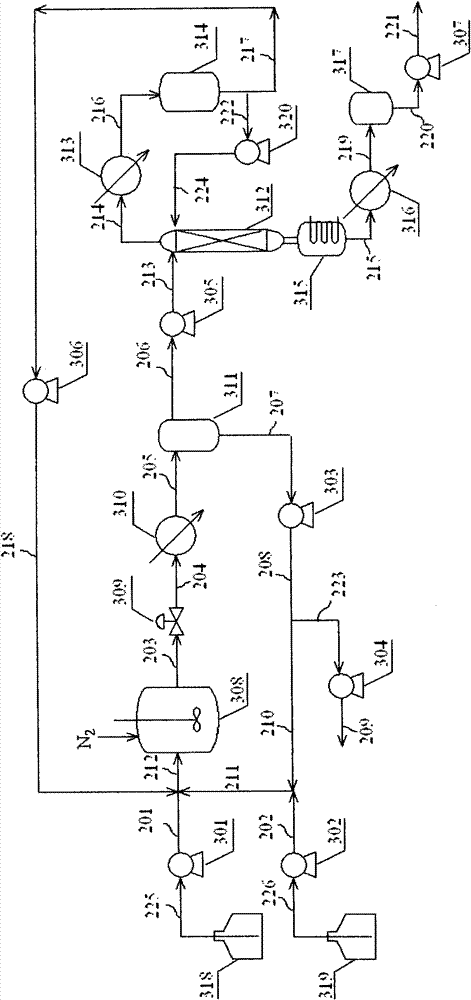
[0078] The reaction process of embodiment 1-3 is in such as figure 2 Carried out in the shown device, the n-butyraldehyde (99.8mol% n-butyraldehyde, 0.1mol% n-butanol, 0.1mol% heavy component) in the storage tank 318 is transported into by the speed of 150ml / hr by pump 301 with stream 225 Reactor, the aqueous solution of catalyst NaOH in storage tank 319 is pressurized by pump 302 with stream 226, and the aqueous solution 202 of catalyst NaOH after pressurization is mixed with the aqueous catalyst solution 210 of circulation to form mixed liquid 211, and feeds 201 with n-butyraldehyde and The 218 from the pump 306 is mixed to form a mixture 212 and then enters the reactor 308. The reactor adopts a 100ml high-pressure stirred tank, and the reaction temperature is maintained at 120 ° C. The pressure of the reactor is controlled at 0.4 by passing a stream of nitrogen into the reactor. MPa, the liquid phase volume in the reactor is controlled at 60 ml by the height position of th...
Embodiment 4
[0079] Embodiment 4 uses a flash tank instead of a distillation tower, and the rest of the equipment is the same as in Embodiment 1-3.
[0080] Table 1 is the operating conditions of the distillation zone of Examples 1-4.
[0081] Distillation equipment operating conditions of each embodiment of table 1
[0082]
[0083] Table 2-3 is some main logistics analysis results of Examples 1-4.
[0084] Table 2 embodiment 1-4 part logistics analysis result
[0085]
[0086] Light fractions are components with a boiling point higher than n-butanol but lower than octenal, and heavy fractions are components with a boiling point higher than octenal.
[0087] Table 3 embodiment 1-4 part logistics analysis result
[0088]
Embodiment 5
[0090] Use valeraldehyde instead as the condensation raw material 201, and deliver n-valeraldehyde (n-valeraldehyde 97%, 2-methylbutyraldehyde 3%) to reactor 308 with a flow rate of 70ml / hr through pump 301, and the reactor pressure is controlled at about 10bar , the pump 304 discharges the reaction product water 309 at a rate of 8 ml / hr. The distillation zone adopts the same flash tank as in Example 4, the operating pressure is 0.1 bar, the temperature is 95° C., and the rest of the operating conditions are the same as in Example 4. The analysis results of some major streams are shown in Table 4.
[0091] Table 4 embodiment 5 part logistics analysis results
[0092]
[0093]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com