Hydroformylation process
A catalyst, phosphinomethyl technology, applied in the field of hydroformylation, can solve the problems such as the increase of the ratio of HBA:HMPA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
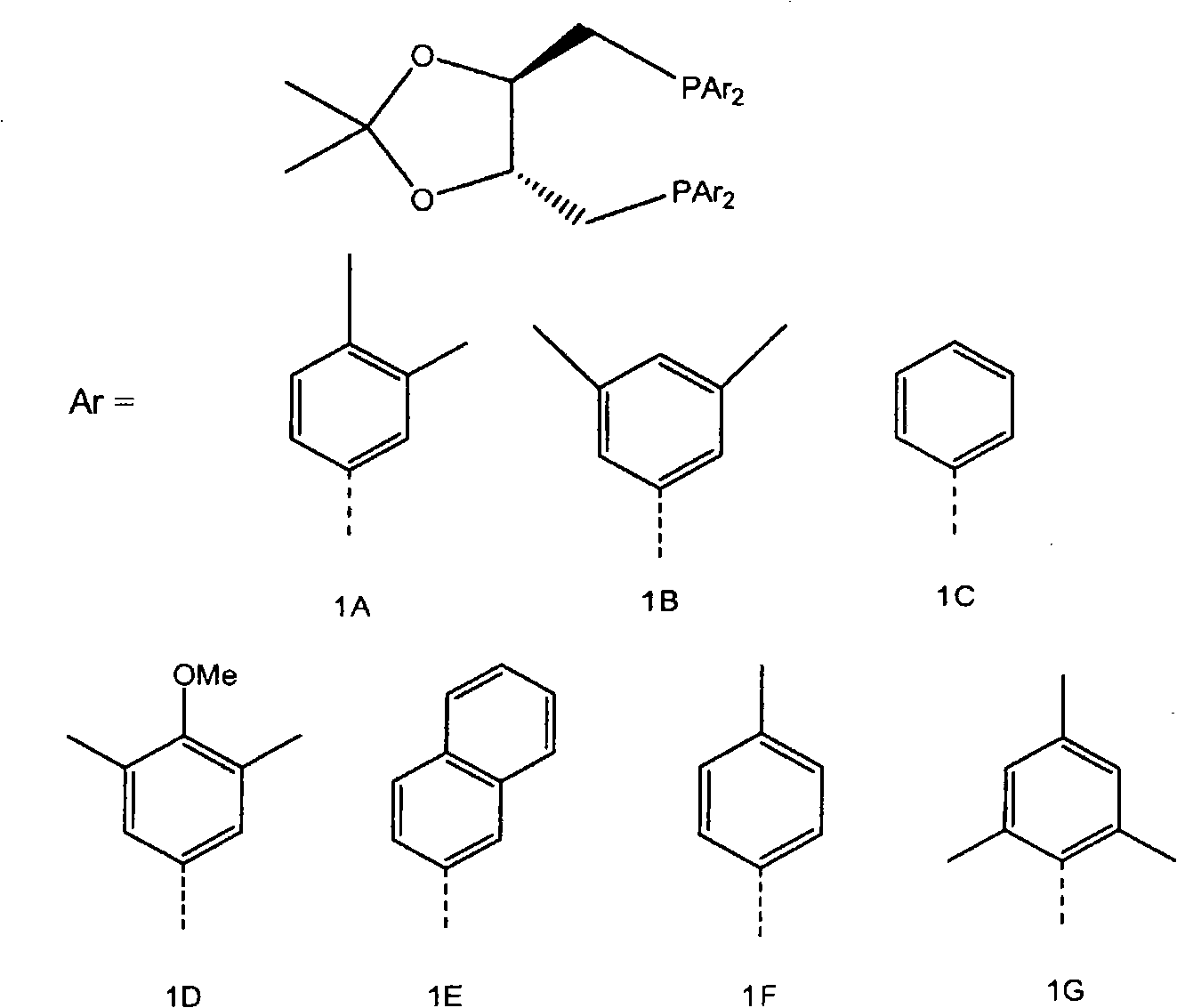
[0031] Embodiment 1: the preparation of diphosphine
[0032] 1A, 1B, 1C, 1D, and 1E : Diphosphines 1A, 1B, 1C, 1D and 1E of the general formula below were prepared as described below.
[0033]
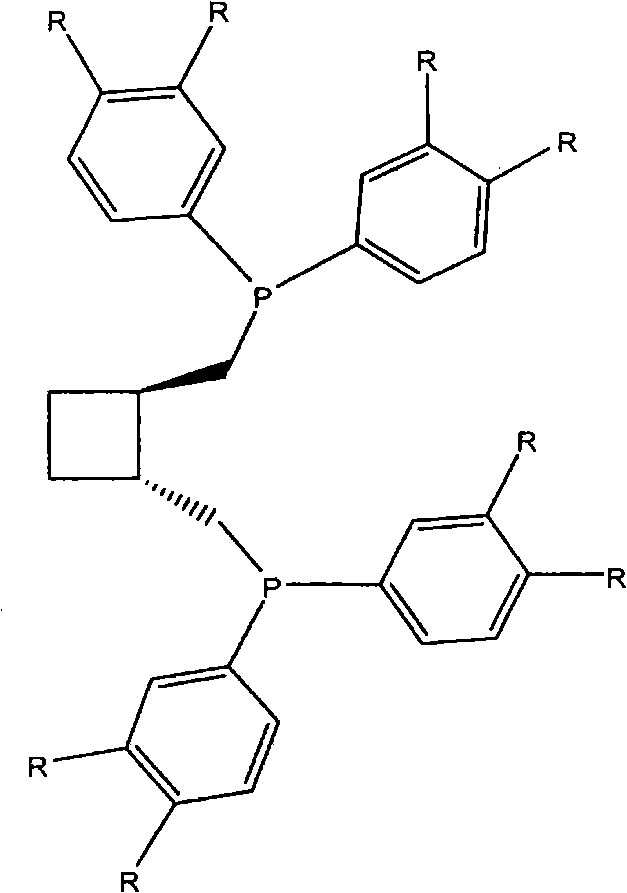
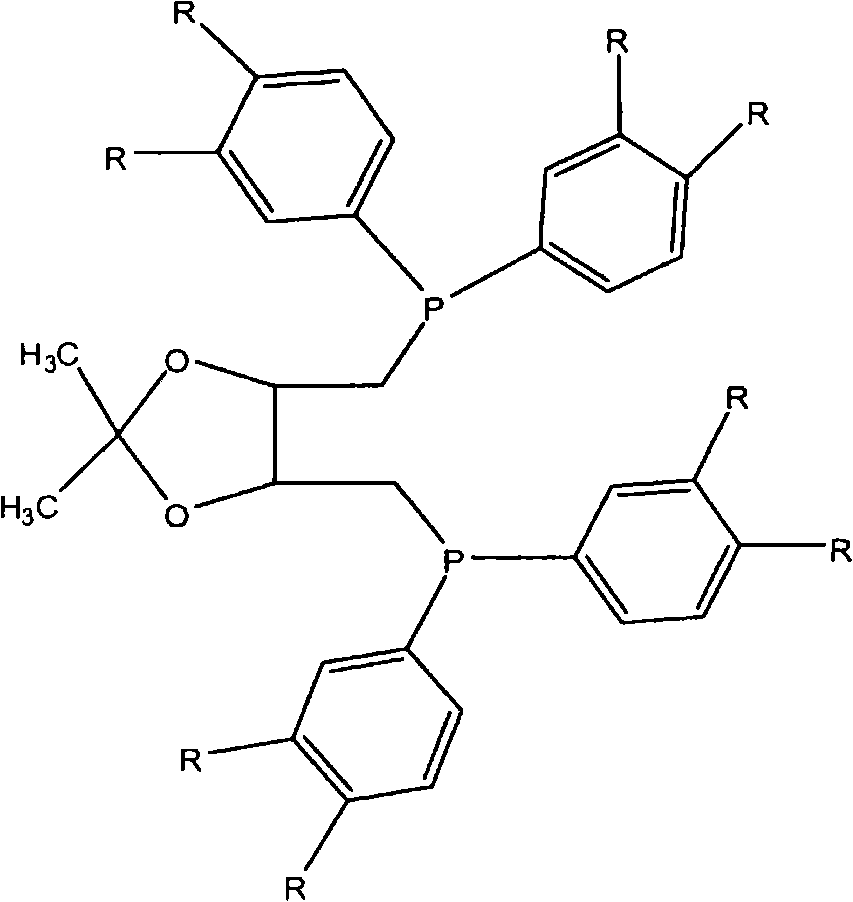
[0034] A solution of 2,2-dimethyl-4,5-bis[(tosyloxymethyl)methyl]-1,3-dioxolane in dry / degassed THF under argon (1 equivalent, 1.73g, 3.7×10 -3 moles of dioxolane in 50 ml THF) was added dropwise to a solution of an appropriate amount of lithium diarylphosphine (see formula above) in dry / degassed THF (2.3 equivalents in 100 ml THF). The mixture was heated to reflux for 2 hours, then cooled, and the solvent was removed under reduced pressure. The remaining solid was redissolved in dichloromethane, filtered through a bed of silica, and the solvent was removed under reduced pressure to give 2,3-O-isopropylidene-2,3-dihydroxy-1,4- Bis(bisarylphosphine)butane.
[0035] Diphosphine 1A: 2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis[bis(3,4-dimethylphenyl)phosphino]butane.
[0036] C...
Embodiment 2
[0042] Example 2: Hydroformylation using a diphosphine
[0043] Hydroformylation of allyl alcohol using diphosphine 1A-1E according to the following procedure:
[0044] The required diphosphine (2 equivalents or 8.6×10 -5 mol) in dry degassed toluene (15 g) was added to [Rh(CO)2(acac)] (1 equivalent or 4.3×10 -5 Moore). Use 1:1 CO / H 2 The solution was rinsed three times with the CO / H 2 The mixture was pressurized to 180 psig. Then the autoclave was heated to 65°C under stirring, allyl alcohol (3.5ml) was injected, and the autoclave was heated with CO / H 2 Mixture The autoclave was pressurized to 200 psig. The autoclave was maintained at a constant pressure of 200 psig and the reaction was monitored for gas uptake. When there was no further gas uptake, the autoclave was cooled and depressurized. The resulting reaction solution was analyzed by gas chromatography to identify the reaction product. This reaction produces HBA, HMPA, and C 3 Products (n-propanol and propiona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com