Stable cetrorelix medicinal composition and preparation method thereof
A technology for cetrorelix and composition, which is applied in the field of polypeptide pharmaceutical compositions and their preparation, can solve the problems of difficult filtration, loss of content, inability to prepare lyophilized pharmaceutical compositions of trorelix, etc., and achieves convenient transportation and process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
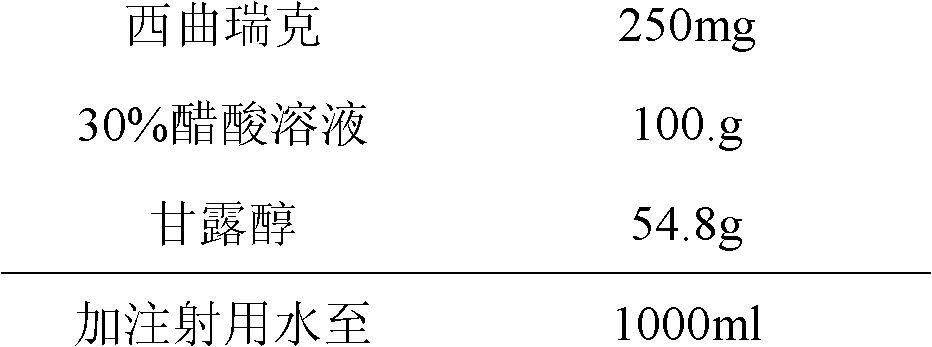
[0022] Prescription (1000 sticks)
[0023]
[0024] Weigh the raw and auxiliary materials according to the prescription amount, dissolve cetrorelix with 30% acetic acid solution, add water for injection and mix well. Then add mannitol to dissolve and mix well, and continue to add injection to the total volume. 0.2μm filter, with 1.0ml / package.
[0025] Lower the temperature of the plate layer to -25°C at 0.5°C / min and keep it warm for 2 hours. Then continue to cool down, let the product drop below -40°C, and keep it warm for 3 hours. Turn on the vacuum and start heating until the product is completely dry.
Embodiment 2
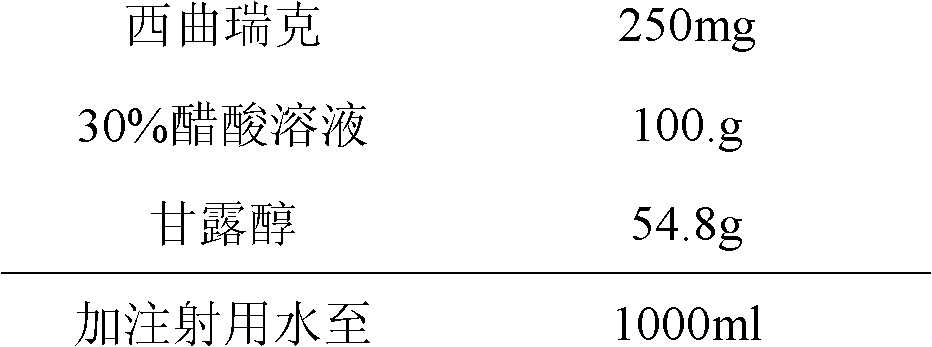
[0027] Prescription (1000 sticks)
[0028]
[0029] Weigh the raw and auxiliary materials according to the prescription quantity, dissolve the cetrorelix with 30% acetic acid solution, add water for injection and mix well, then add mannitol to dissolve and mix well. Continue adding water for injection to total volume. 0.2μm filter, with 1.0ml / package.
[0030] Reduce the temperature of the plate layer to -40°C at 1°C / min and keep it warm for 1.5 hours. Then continue to cool down, let the product drop below -40°C, and keep it warm for 2 hours. Turn on the vacuum and start heating until the product is completely dry.
Embodiment 3
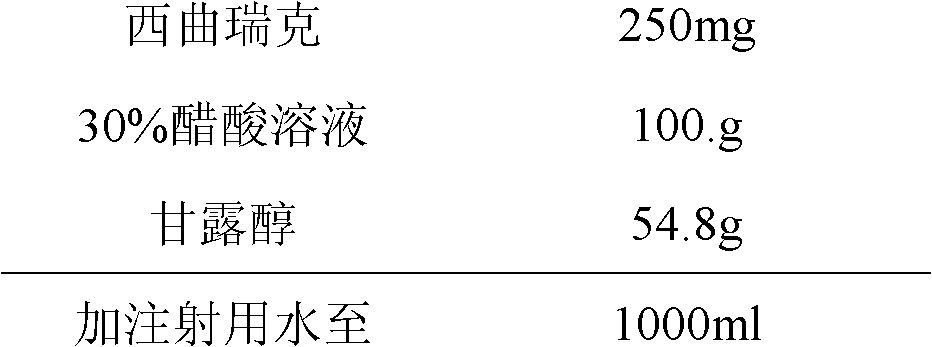
[0032] Prescription (1000 sticks)
[0033]
[0034] Lower the temperature of the plate layer to -37°C at 1°C / min and keep it warm for 1.5 hours. Then continue to cool down, let the product drop below -40°C, and keep it warm for 2 hours. Turn on the vacuum and start heating until the product is completely dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com