Nonaqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, which is applied in the direction of non-aqueous electrolyte battery electrodes, secondary batteries, battery electrodes, etc., can solve the problems of self-discharge and side reactions easily, and achieve the effect of self-discharge inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] First, lithium iron phosphate (LiFePO 4 ) powder 91% by weight, acetylene black 2.5% by weight, graphite 3% by weight, and polyvinylidene fluoride (PVdF) 3.5% by weight, were mixed to prepare a slurry. The slurry was coated on an aluminum foil (current collector) with a thickness of 15 μm, dried and then pressed to produce a 3 The positive electrode of the positive electrode layer.
[0058]
[0059] First, spinel lithium-titanium composite oxide (Li 4 Ti 5 o 12 ) powder 85% by weight, graphite 5% by weight, acetylene black 3% by weight, and PVdF 7% by weight, and these were mixed to prepare a slurry. The slurry was coated on an aluminum foil (collector) having a thickness of 11 μm, dried and pressed to produce a film having a density of 2.0 g / cm 3 The negative electrode of the negative electrode layer.
[0060]
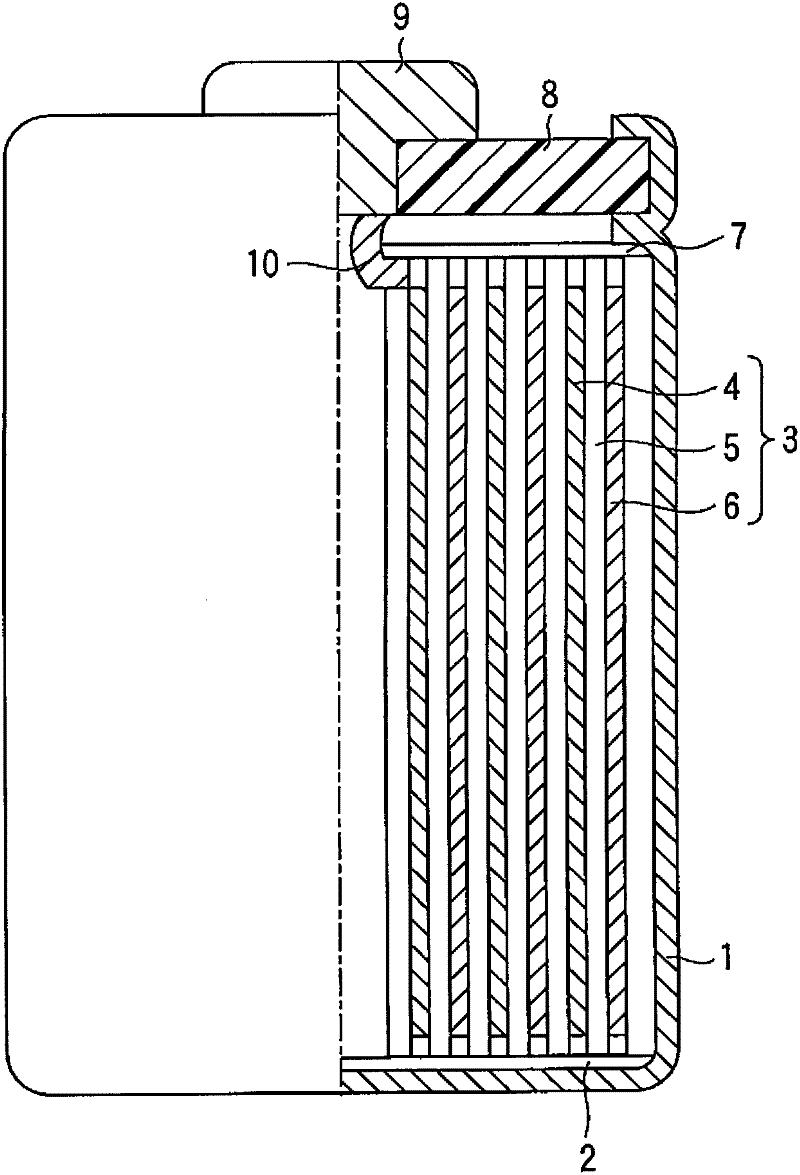
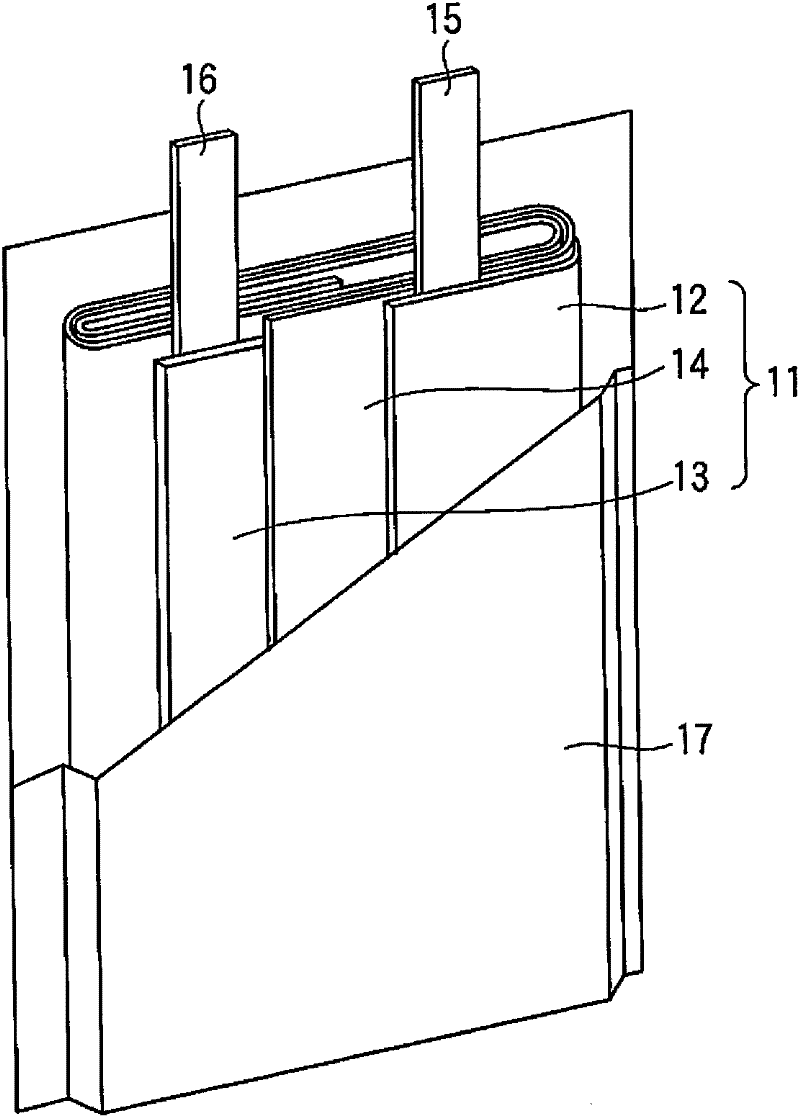
[0061] The positive electrode, the separator made of a polyethylene porous film, the negative electrode, and the separator were stacked in...
Embodiment 2~10
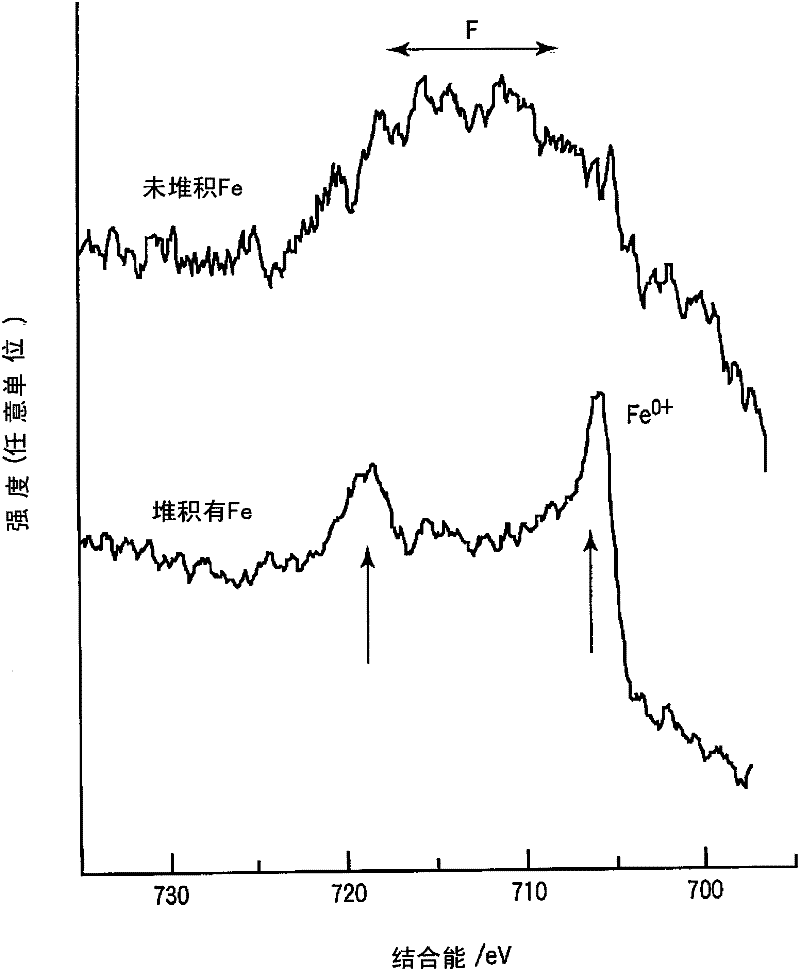
[0072] A secondary battery similar to that of Example 1 was produced except that the aging treatment described in Table 1 was performed. XPS, AES, and photographs of the negative electrode were taken in the same manner as in Example 1. As a result, the same deposition form of metallic iron on the negative electrode as in Example 1 was confirmed. In addition, the results of the proportion of metallic iron covered on the surface of the negative electrode layer, the number of metallic iron non-covered regions, and the maximum height of the covered region calculated in Example 1 are also listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com