Chitosan quaternary amine salt gallate, synthetic method thereof and application thereof
A technology of acid chitosan quaternary ammonium salt ester and gallic acid, which is applied in the field of daily chemicals, can solve the problems of poor thermal stability, low molecular weight, and low anti-oxidation efficiency of gallic acid, achieve easy availability of equipment and raw materials, and enhance biological activity , a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
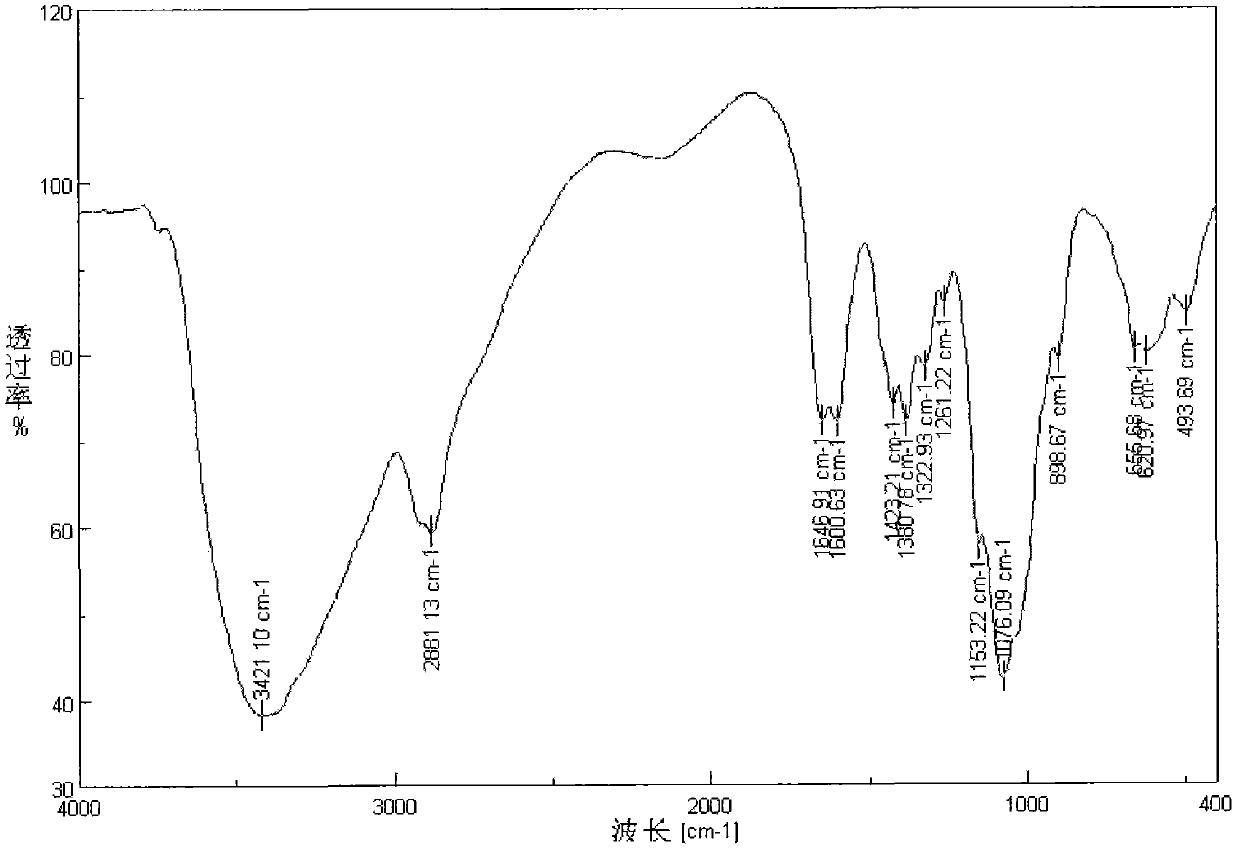
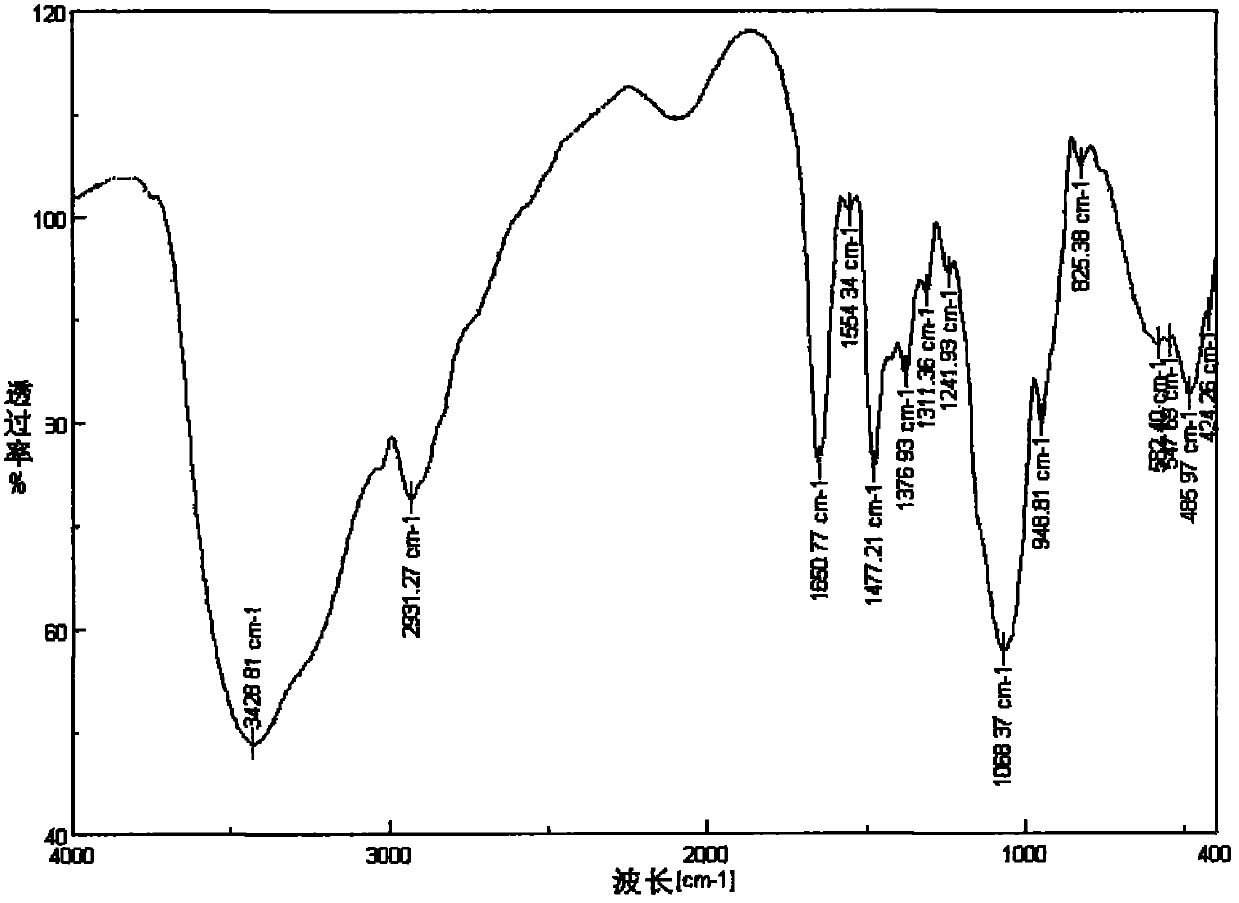
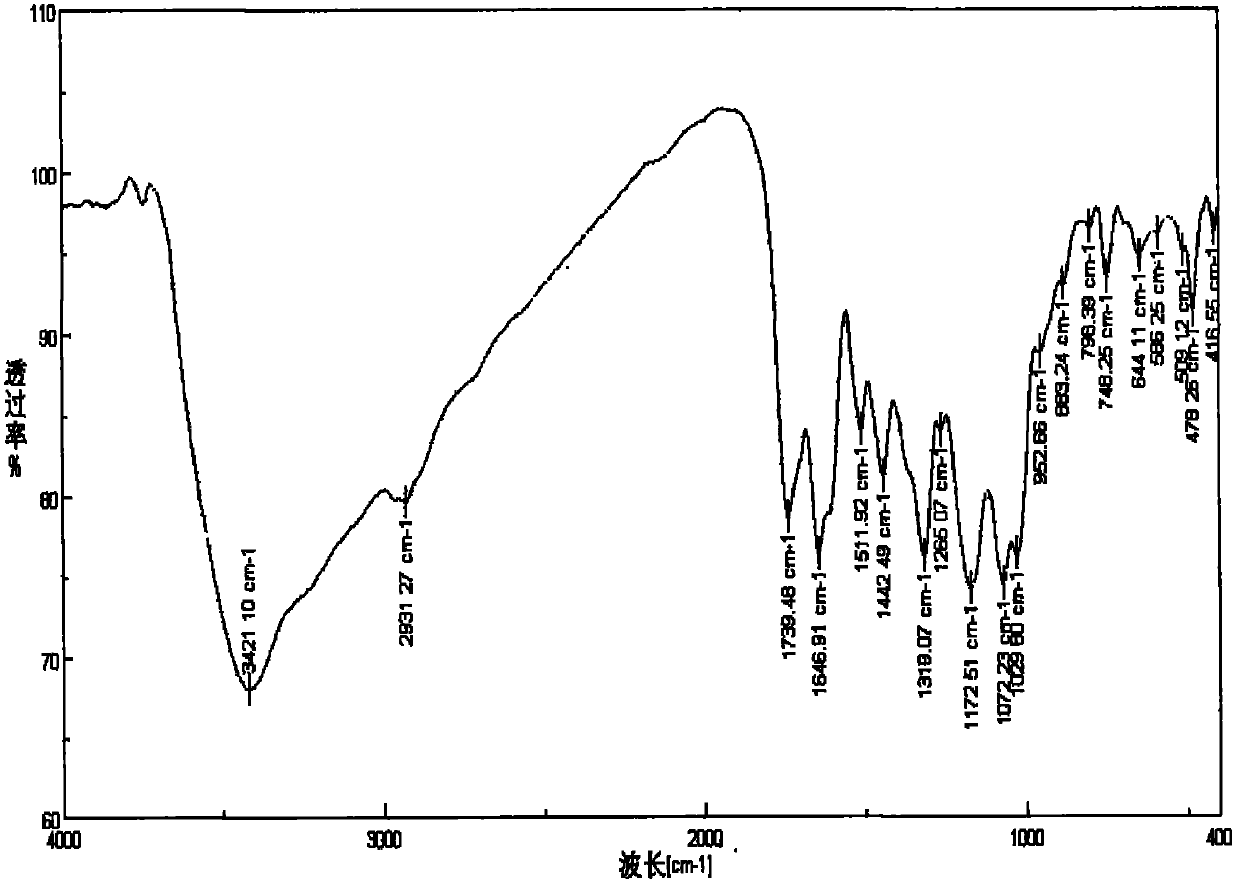
Image
Examples
Embodiment 1
[0018] Chitosan gallic acid trimethyl quaternary ammonium salt ester is the compound shown in (formula 1)
[0019]
[0020] Synthesis of gallic acid chitosan trimethyl quaternary ammonium ester:
[0021] (1) Under the protection of nitrogen, 3.4g gallic acid and 2mL thionyl chloride were refluxed in tetrahydrofuran solvent for 2 hours, and the solvent and excess thionyl chloride were distilled off under reduced pressure to obtain acylated gallic acid;
[0022] (2) Dissolve 2g chitosan trimethyl quaternary ammonium salt in 100mL N-methylpyrrolidone solvent.
[0023] (3) The acylated gallic acid in the step (1) was mixed with the chitosan trimethyl quaternary ammonium salt solution obtained in the step (2) in an ice bath, and the resulting reaction solution was reacted at room temperature for 8 h. After the reaction was finished, the reaction solution was poured into 600 mL of acetone, and a precipitate was precipitated. The precipitate was washed successively with acetone ...
Embodiment 2
[0029] The difference from Example 1 is:
[0030] (1) Under nitrogen protection, reflux 2 g of gallic acid and 2 mL of thionyl chloride in tetrahydrofuran solvent for three hours, then distill under reduced pressure to remove excess thionyl chloride and solvent to obtain acylated gallic acid.
[0031] (2) After mixing 2 g of chitosan trimethyl quaternary ammonium salt with acylated gallic acid obtained in the first step in 100 mL of N-methylpyrrolidone solvent, they were stirred and reacted at -3° C. for 10 hours.
[0032] (3) After the reaction, the obtained product is precipitated with acetone, washed with acetone and ethanol in sequence, and then freeze-dried under vacuum to obtain the product.
Embodiment 3
[0034] The difference from Example 1 is:
[0035] 1) Under the protection of nitrogen, reflux 1.7 g of gallic acid and 2 mL of thionyl chloride in tetrahydrofuran solvent for three hours, then distill under reduced pressure to remove excess thionyl chloride and solvent to obtain acylated gallic acid.
[0036] (2) After mixing 1 g of chitosan trimethyl quaternary ammonium salt with acylated gallic acid obtained in the first step in 100 mL of N-methylpyrrolidone solvent, they were stirred and reacted at 0° C. for 16 hours.
[0037] (3) After the reaction, the obtained product is precipitated with acetone, washed with acetone and ethanol in sequence, and then freeze-dried under vacuum to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com