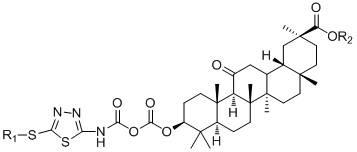
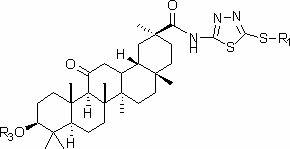
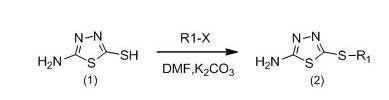
Glycyrrhetinic acid derivatives and preparation method, medicinal composition and application thereof to preparation of antitumor drug
A technology of glycyrrhetinic acid and glycyrrhetinic acid ester, which is applied in the field of compounds and can solve the problems of weak anti-tumor activity of glycyrrhetinic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0028] Preparation of 3-{[(5-methylthio-1,3,4-thiadiazole-2)amino]-1,4-dioxobutoxy}glycyrrhetinic acid methyl ester.
[0029] a), Preparation of 2-amino-5-methylmercapto-1,3,4-thiadiazole.
[0030] Weigh 2.0g (14mmoL) of 2-mercapto-5-amino-1,3,4-thiadiazole, add 20 mL of anhydrous dimethylformamide (DMF), then add 2.76g of anhydrous K 2 CO 3 , stirred at room temperature for 10 min, finally added 2.82g (20mmoL) methyl iodide, continued the reaction for 4h, stopped the reaction, poured the reaction solution into 50ml of ice water, and left it overnight, a white solid precipitated, suction filtered, and the filter cake was weighed with absolute ethanol Crystallized to obtain 1.91g of white crystals, yield 93%, mp: 158-160°C.
[0031] b) Preparation of methyl 3-O-succinic acid monoacylglycyrrhetinate.
[0032] Add 0.96g (2.00mmol) methyl glycyrrhetinate, 0.40g (4.00mmol) succinic anhydride, 0.048g (4.00mmol) 2-aminopyridine (DMAP) into 50ml of anhydrous dichloromethane, stir a...
preparation Embodiment 2
[0036] Preparation of 3-{[(5-ethylthio-1,3,4-thiadiazole-2)amino]-1,4-dioxobutoxy}glycyrrhetinic acid methyl ester.
[0037] a), Preparation of 2-amino-5-ethylmercapto-1,3,4-thiadiazole.
[0038] Referring to the preparation method of step a in Preparation Example 1, it is prepared by reacting bromoethane with 2-mercapto-5-amino-1,3,4-thiadiazole, white solid, yield 88%, mp: 152~ 154°C.
[0039] b), Preparation of 3-O-succinic acid monoacyl glycyrrhetinate methyl ester.
[0040] The difference between the preparation method of this step and the preparation method of step b in Preparation Example 1 is that the reaction solution is concentrated under reduced pressure and then recrystallized with water, and the rest are the same.
[0041] c), Preparation of 3-{[(5-ethylthio-1,3,4-thiadiazole-2)amino]-1,4-dioxobutoxy}glycyrrhetinic acid methyl ester
[0042] Referring to the preparation method of step c in Preparation Example 1, the 2-amino-5-ethylmercapto-1,3,4 prepared fro...
preparation Embodiment 3
[0044] 3-{[(5-(tert-butoxy-carbonyl-methylthio)-1,3,4-thiadiazole-2)amino]-1,4-dioxobutoxy}glycyrrhetinic acid A Esters (I 3 ) preparation.
[0045] a), Preparation of 2-amino-5-(tert-butoxycarbonylmethylthio)-1,3,4-thiadiazole.
[0046] Referring to the preparation method of step a in Preparation Example 1, it was prepared by reacting tert-butyl bromoacetate with 2-mercapto-5-amino-1,3,4-thiadiazole, white solid, yield 45%, mp: 105-107°C.
[0047] b), Preparation of 3-O-succinic acid monoacyl glycyrrhetinate methyl ester.
[0048] The preparation method of this step is the same as the preparation method of step b in Preparation Example 1.
[0049] c), 3-{[(5-(tert-butoxy-carbonyl-methylthio)-1,3,4-thiadiazole-2)amino]-1,4-dioxobutoxy}licorice Methyl hypochlorite (I 3 ) preparation.
[0050] Referring to the preparation method of step c in Preparation Example 1, the 2-amino-5-(tert-butoxycarbonylmethylthio) prepared in step a of 3-O-succinic acid monoacyl glycyrrhetinat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com