Magnetic immunochromatography for quickly detecting L. monocytogenes and preparation of test strip for detection
A technology of Listeria monocytogenes and magnetic immunochromatography, which can be applied to measurement devices, analytical materials, instruments, etc., can solve the problems of inability to detect Listeria monocytogenes quickly and easily, and achieve high sensitivity and short time consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] A. Preparation and purification of antibodies:
[0042] The UV-inactivated Listeria monocytogenes was used as the antigen to immunize BALB / C mice. The monoclonal antibody cell lines were prepared and screened according to the conventional hybridoma technology and limiting dilution method, and then the specificity of the anti-Listeria monocytogenes The antibody cell line is proliferated and cultured, and injected into BALB / C mice to prepare ascites. After the prepared ascites is concentrated by 50% weight / volume ratio of ammonium sulfate, the antibody is purified using a commercial Protein-G affinity chromatography column; When specifically performing affinity chromatography purification, follow the attached product operating instructions.
[0043] The UV-inactivated Listeria monocytogenes was used as the antigen to immunize New Zealand white rabbits. The resulting antiserum was concentrated by 50% weight / volume ratio of ammonium sulfate, and then desalted using a commercial ...
Embodiment example 1
[0058] Implementation case 1 Preparation of test strips for magnetic detection of Listeria monocytogenes
[0059] (1) Preparation of specific immunomagnetic beads for Listeria monocytogenes
[0060] EDC / NHS coupling
[0061] The Listeria monocytogenes specific murine monoclonal antibody is coupled with carboxyl modified nanomagnetic beads (with a particle size of 200nm) to prepare Listeria monocytogenes specific immunomagnetic beads, that is, magnetic labeled antibodies. Take the pH5.0 MEST solution (0.05% Tween-20) as the activation buffer solution, take 2mg of carboxyl magnetic beads into a 2mL centrifuge tube, add 500μL activation buffer, mix well on the vortex shaker, and then place the centrifuge tube in On the magnetic separation rack, after the magnetic beads are completely adsorbed, extract the supernatant with a miniature desktop vacuum pump; after re-washing the magnetic beads twice with 500μL activation buffer, adjust the volume of EDC and NHS solution to 500μL with activ...
Embodiment example 2
[0065] Implementation of case 2 qualitative testing of samples
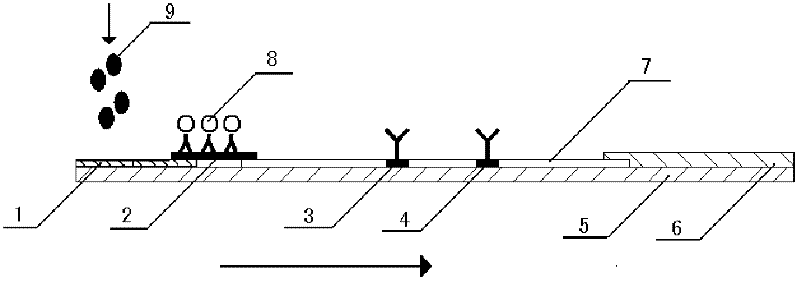
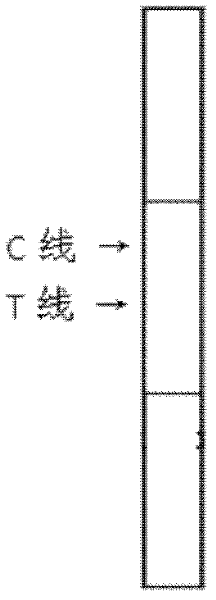
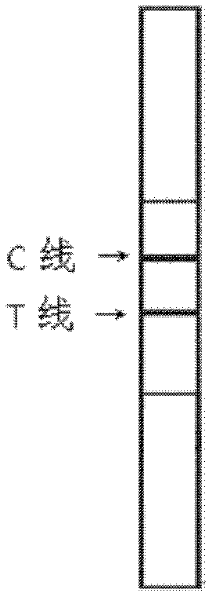
[0066] Add 100 μL of sample to the sample pad of the test strip, and after reacting at room temperature for 20 minutes, observe the test result with naked eyes. See figure 2 , The positive result shows two obvious bands or the T-line band is lighter than the C-line band, while the negative result only shows one band at the C-line, and there is no band at the T-line. If there is no band at the C line during the test, there is a problem with the test strip system, and the test result is deemed invalid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com