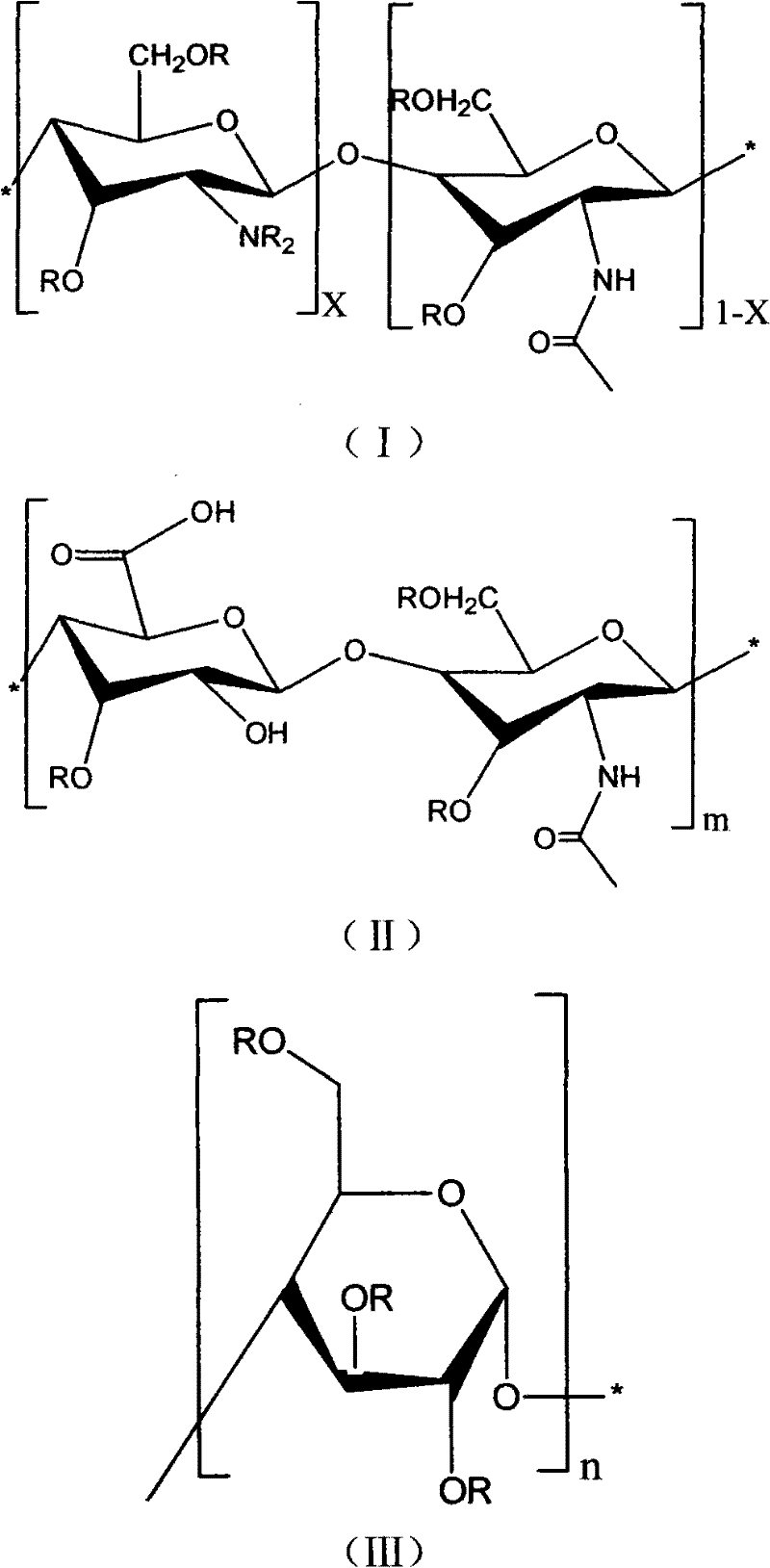
Natural high-molecular acrylate and its preparation method
A natural polymer and acrylate technology, which is applied in the field of natural polymer acrylate derivatives and their preparation, can solve the problems of many reaction steps, intense reaction conditions, and poor water solubility of products, and achieve mild conditions and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] With 2.0g chitosan (DP=90%, Mw=300) with the volume ratio of 200mL be in the solution of triethylamine and chloroform of 1: 1, stir at room temperature and place 48 hours in the ice of 5 ℃ Cool and stir in the bath for 2 hours, dissolve 4.0g of acryloyl chloride in 10mL of chloroform and stir evenly, slowly drop into the chitosan solution in an ice bath and react for 4 hours, continue to react at room temperature for 8 hours, The filtered precipitate was washed three times with acetone to obtain the natural biological material derivative.
[0019] Dissolve 5.0 g of the synthesized natural polymer derivative in 400 mL of phosphate buffer solution with pH=7.4, add 2.02 g of photoinitiator 2-hydroxy-2-methyl-1-p-hydroxyethyl ether phenylacetone , and stir until the solution is uniform. Then inject in the polytetrafluoroethylene template of 8 millimeters (diameter) * 1 millimeter (thickness), after upper and lower two sides are clamped with glass slide glass, leave standst...
Embodiment 2
[0021] With 2.0g chitosan (DP=92%, Mw=3000) with the volume ratio of 300mL is in the solution of diethylamine and dichloromethane of 2: 1, stir at room temperature and place 48 hours in the ice of 8 ℃ Cool and stir in the bath for 2 hours, dissolve 5.0g of methacryloyl chloride in 12.5mL of dichloromethane and stir evenly, slowly add it to the chitosan solution in an ice bath at 1°C to react for 4 hours, and continue at room temperature The reaction was carried out for 8 hours, and the filtered precipitate was washed three times with acetone to obtain the natural biological material derivative.
[0022] Dissolve 5.0 g of the synthesized natural polymer derivative in 400 mL of phosphate buffer solution with pH=7.4, add 2.02 g of photoinitiator 2-hydroxy-2-methyl-1-p-hydroxyethyl ether phenylacetone , and stir until the solution is uniform. Then inject in the polytetrafluoroethylene template of 8 millimeters (diameter) * 1 millimeter (thickness), after upper and lower two sides...
Embodiment 3
[0024] Add 2.0 g of hyaluronic acid (Mw=3000) to 300 mL of a solution of triethylamine and chloroform with a volume ratio of 2:1, stir at room temperature for 48 hours, then cool and stir in an ice bath at 3°C for 2 6.0 g of acryloyl chloride was dissolved in 20 mL of chloroform and stirred evenly, slowly added dropwise to the solution of hyaluronic acid in an ice bath to react for 4 hours, heated to 30°C and reacted for 8 hours, and the precipitate after filtration Wash with acetone three times to obtain the natural biological material derivative.
[0025] Dissolve 5.0 g of the synthesized natural polymer derivative in 400 mL of phosphate buffer solution with pH=7.4, add 2.02 g of photoinitiator 2-hydroxy-2-methyl-1-p-hydroxyethyl ether phenylacetone , and stir until the solution is uniform. Then inject in the polytetrafluoroethylene template of 8 millimeters (diameter) * 1 millimeter (thickness), after upper and lower two sides are clamped with glass slide glass, leave st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com