2,6-dipicolinic acid-(ferrocene monoformic acid)triphenyltin complex, its preparation method and its application
A technology of dipicolinic acid and triphenyltin, applied in drug combination, antineoplastic drugs, organic chemistry, etc., to achieve high anticancer activity, simple preparation method and high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
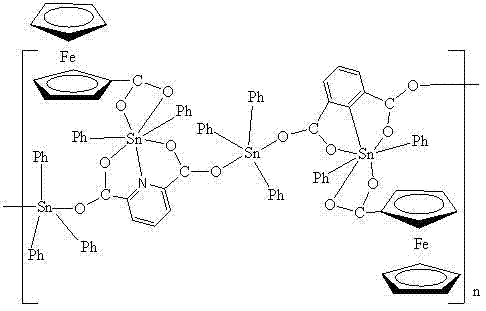
[0017] Example 1: 1.0 mmol FcCOOH (ferrocene monocarboxylic acid), 1.0 mmol NEt 3 (Triethylamine) was dissolved in 15ml of methanol, and then the solution was slowly dropped into 2.0 mmol Ph 3 In the methanol solution of SnCl (triphenyltin chloride), stir at room temperature for 0.5 h, then add 1.0 mmol of 2,6-pyridinedicarboxylic acid dropwise into the above mixed solution, and continue stirring at room temperature for 20 h. Filter and let the filtrate stand at room temperature. After three months, red blocky crystals were obtained, namely organotin compounds 1, The compound yield was 86.5%. The resulting organotin compound , Through infrared spectrum analysis and X-single crystal diffraction analysis, the results are as follows: IR (KBr, cm -1 ): υ = 2984, 2930 (Cp), 1591, 1474 (COO), 1430 (C=N), 795, 686(Sn-O), 509(Sn-C).
[0018] Crystallographic data: The crystal system of the compound belongs to the monoclinic system, the space group is P2(1) / c, and the unit cell p...
Embodiment 2
[0019] Example 2: 1.0 mmol FcCOOH (ferrocene monocarboxylic acid), 1.5 mmol NEt 3 (Triethylamine) was dissolved in 15ml of methanol, and then the solution was slowly dropped into 2.0 mmol Ph 3 In the methanol solution of SnCl (triphenyltin chloride), stir at room temperature for 0.5 h, then add 1.0 mmol of 2,6-pyridinedicarboxylic acid dropwise into the above mixed solution, and continue stirring at room temperature for 21 h. Filter and let the filtrate stand at room temperature. After three months, red blocky crystals were obtained, and the yield of the compound was 87.3%.
Embodiment 3
[0020] Example 3: 1.0 mmol FcCOOH (ferrocene monocarboxylic acid), 2.0 mmol NEt 3 (Triethylamine) was dissolved in 15ml of methanol, and then the solution was slowly dropped into 2.0 mmol Ph 3 In the methanol solution of SnCl (triphenyltin chloride), after stirring at room temperature for 0.5 h, 1.0 mmol of 2,6-pyridinedicarboxylic acid was added dropwise to the above mixed solution, and continued to stir at room temperature for 22 h. Filter and let the filtrate stand at room temperature. After three months, red blocky crystals were obtained, and the yield of the compound was 86.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com