Cefoxitin anhydrous crystal, preparation method thereof and method for preparing cefoxitin sodium by using same
A technology for cefoxitin acid and anhydrous crystallization, which is applied in the field of preparation of pharmaceutical compounds, and can solve problems such as incomplete crystallization, high total impurities of cefoxitin acid hydrate, and impact on product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Take 10g of cefoxitin acid hydrate, add it to the reaction flask, raise the temperature to 36°C, add tetrahydrofuran and stir until it dissolves completely, concentrate under reduced pressure to 50-70mL, add dichloromethane dropwise to make the solution cloudy. Cultivate the crystal at 20° C. for half an hour, add dichloromethane to dilute, and continue to grow the crystal at room temperature for half an hour. After filtering, the filter cake was washed with dichloromethane, and the temperature was controlled at 40° C. to dry under vacuum to obtain white crystals of cefoxitin anhydrous. Agilent1200 reversed-phase high-performance liquid phase detection its purity is 99.838% (as Figure 7 Shown), the K-F method measures its water content as 0.28%.
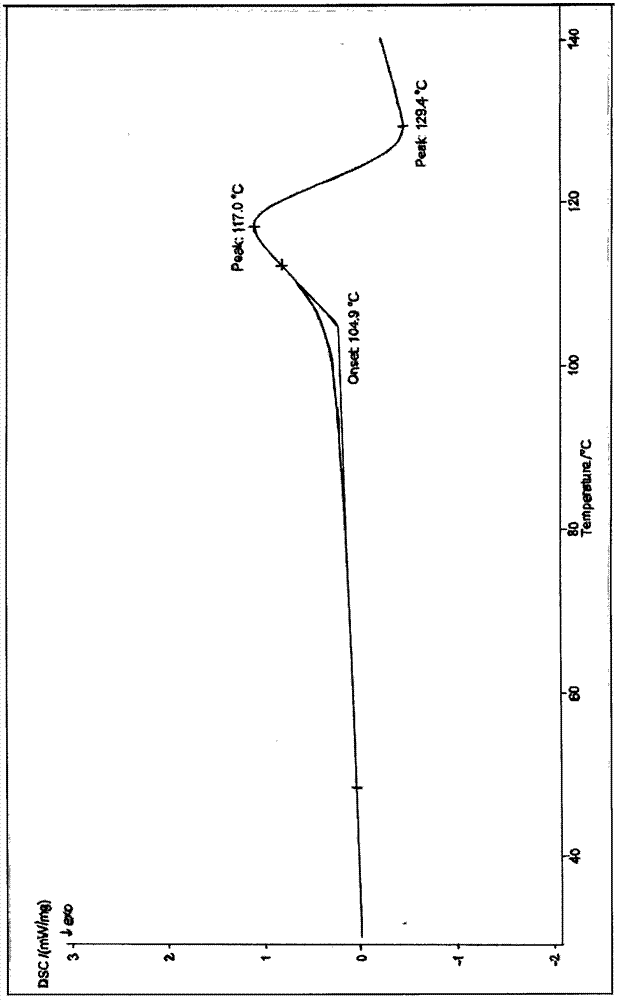
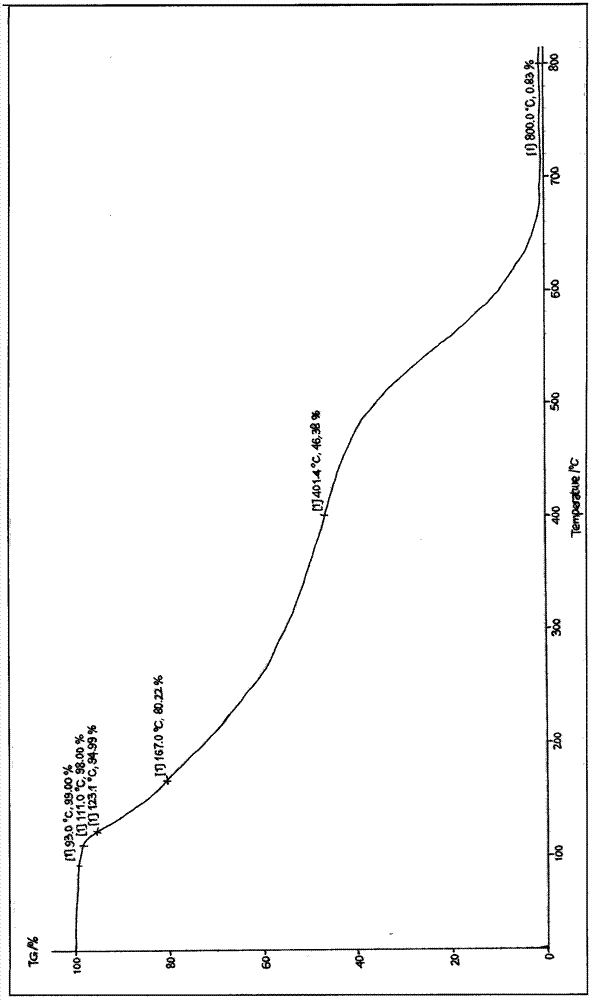
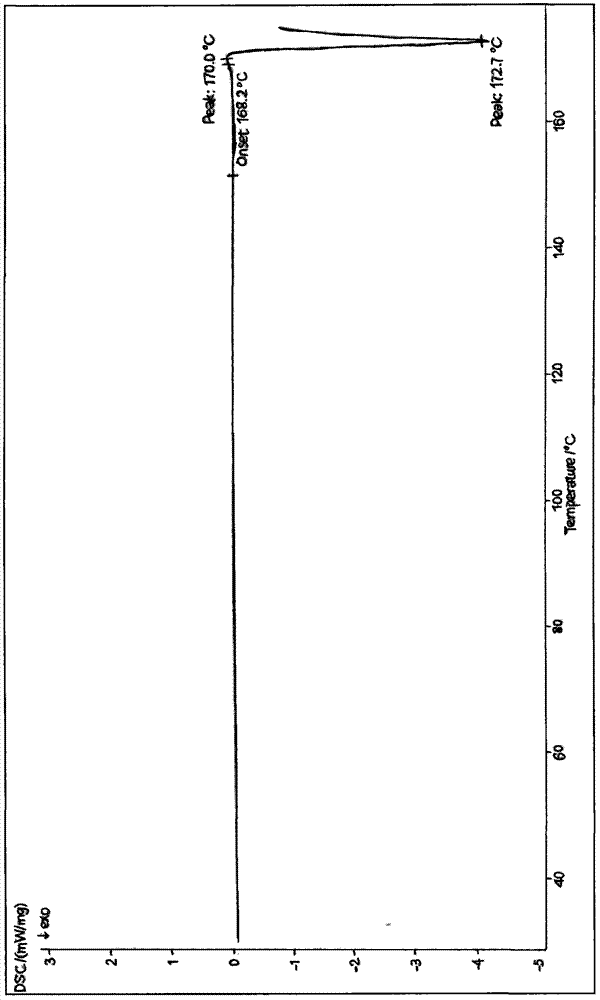
[0046] Gained cefoxitin acid anhydrous crystalline product adopts melting point measuring instrument to measure its melting point to be 152~153 ℃, and its X-ray diffraction pattern is as follows Figure 5As shown, its infra...
Embodiment 2
[0050] Take 10g of cefoxitin acid hydrate, add it to the reaction bottle, raise the temperature to 36°C, add ethanol and stir until it dissolves completely, concentrate under reduced pressure to 50-70mL, add dichloromethane dropwise to make the solution cloudy. Cultivate the crystal at 20° C. for half an hour, add dichloromethane to dilute, and continue to grow the crystal at room temperature for half an hour. After filtering, the filter cake was washed with dichloromethane, and the temperature was controlled at 40° C. to dry under vacuum to obtain white crystals of cefoxitin anhydrous. Agilent1200 reversed-phase high-performance liquid phase detection its purity is 99.747%, and its water content is 0.45% as determined by K-F method.
[0051] The obtained cefoxitin acid anhydrous crystalline product is measured by a melting point detector and has a melting point of 152-153°C. The X-ray diffraction pattern of the product prepared in this example is basically consistent with th...
Embodiment 3
[0053] Take 10 g of the anhydrous cefoxitin acid crystals prepared in the above examples and dissolve them in a mixed solution of 40 mL of methanol and 60 mL of acetone, and add dropwise a solution containing 5.5 g of sodium isooctanoate (sodium 2-ethylhexanoate) at a temperature of about 5° C. and 50mL of methanol, and then add 250mL of acetone to precipitate a solid. The solid is suction filtered, washed with acetone, and dried in vacuo to obtain about 9.6 g of cefoxitin sodium.
[0054] The cefoxitin sodium obtained in this example and the cefoxitin sodium prepared by cefoxitin acid hydrate are measured by the first method of solution color inspection in the Chinese Pharmacopoeia 2010 edition, the color level of the former is less than Y1 and the color level of the latter is less than Y4 . The color level of the cefoxitin sodium prepared from the anhydrous cefoxitin acid crystal of the present invention is significantly reduced, and has a good application prospect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com