Poly-squaric acid conjugated polymer preparation method and application thereof
A technology of conjugated polymers and polysquaric acid, which is applied in the preparation of polysquaric acid conjugated polymers, in the field of polysquaric acid conjugated polymers, can solve the problems of limiting the application of polysquaric acid materials, and achieve good Solubility, broad absorption spectrum, improved capture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
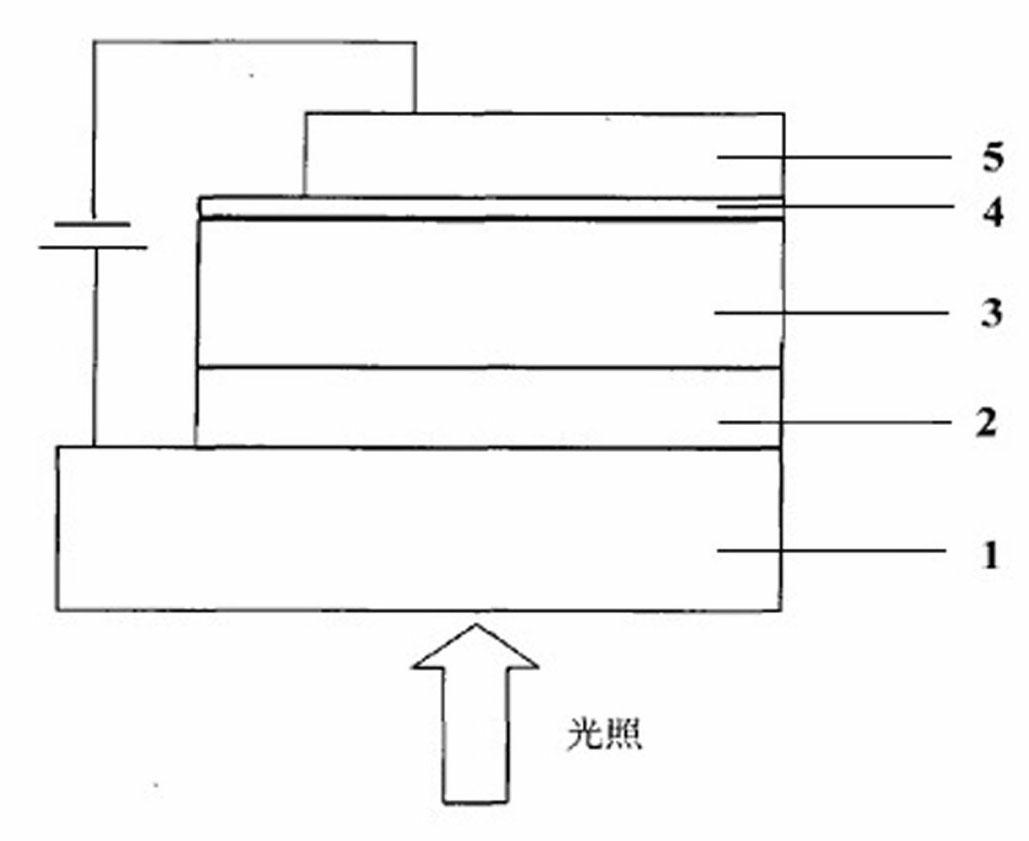
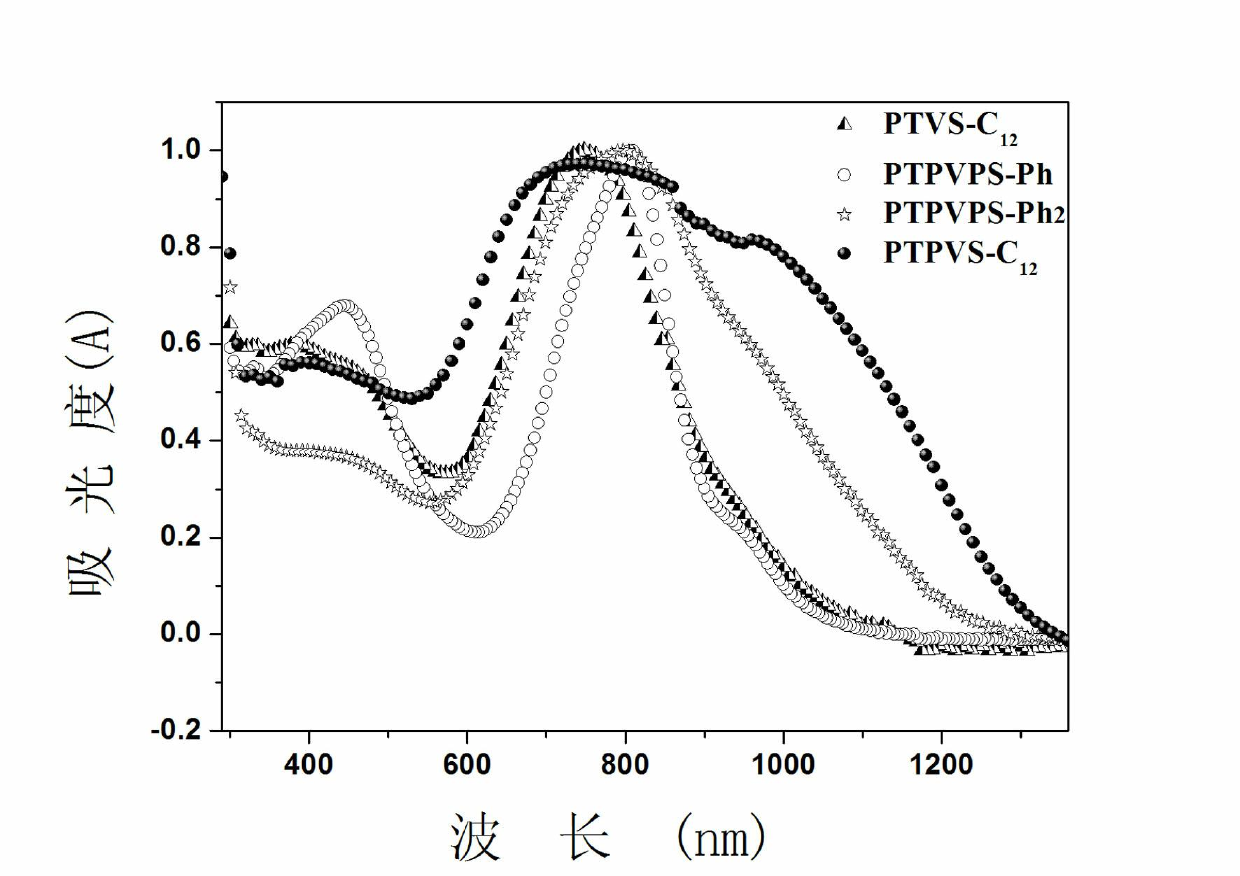
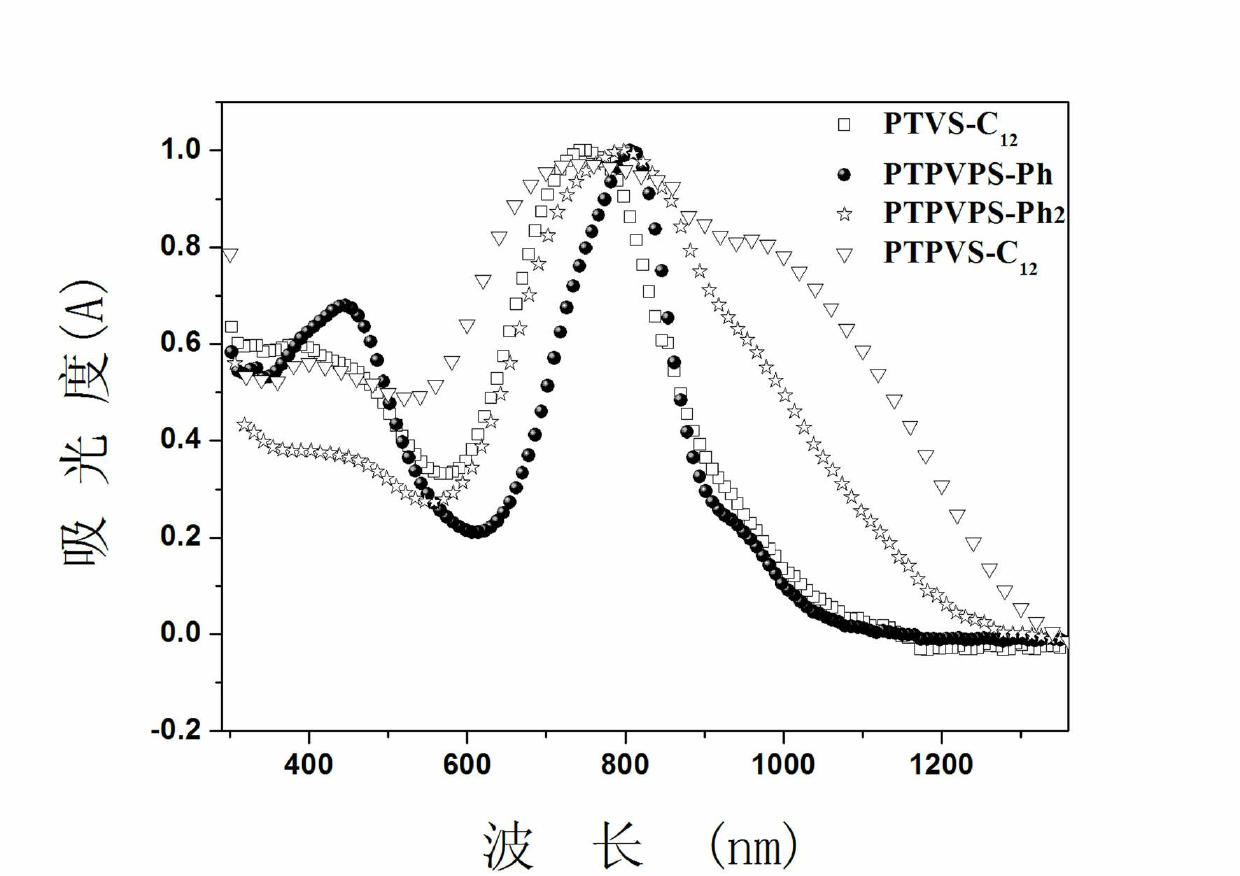
Image
Examples
Embodiment 1
[0039] Example 1 Polymer PVTVPS-Ph 2 preparation of
[0040] (1) Preparation of 3-dodecylthiophene
[0041]
[0042] Metal magnesium (3g, 123.46mmol) was added to a 250mL three-necked flask filled with 30mL of anhydrous ether, and then n-dodecyl bromide (24.92g, 100mmol) was added. Under argon protection, heat and reflux until the reaction of metal magnesium is complete, then add 20mg Ni(dppp)Cl 2 and 3-bromothiophene (16.3g, 100mmol), the mixture was heated to reflux for 12h and then poured into 200mL of ice-cold 2mol / L hydrochloric acid aqueous solution, the organic layer was separated, and the aqueous layer was extracted with ether (30mL×3), the organic phases were combined, and washed with over magnesium sulphate and dried overnight. After filtering off the desiccant, the solvent was distilled off, and then distilled under reduced pressure to obtain 18.40 g of a colorless oily substance of 3-dodecylthiophene, with a yield of 93%. Boiling point: 132°C-135°C / 800Pa [lite...
Embodiment 2
[0058] Embodiment 2: the preparation of PTPVPS-Ph
[0059] (1) Preparation of 4-dodecyloxyphenylboronic acid
[0060] Under the protection of argon, 4-dodecyloxybromobenzene (8.53g, 25mmol) and 80mL of anhydrous tetrahydrofuran were added to a 250mL three-necked flask, cooled to -35°C with liquid nitrogen-acetone, Slowly add 19 mL of n-butyllithium (1.6 mol / L, 30.4 mmol) dropwise, and keep at -35°C for 2 h after the dropwise addition. Cool to -78°C and keep for 2h; start to add 8mL of trimethyl borate dropwise, and keep at -78°C for 20min after the dropwise addition. Rise naturally to room temperature for 24 hours, add 40 mL of 20% hydrochloric acid, stir at room temperature for 1 hour, pour the reaction solution into 100 mL of water, extract with ether, wash with distilled water, and dry overnight over anhydrous magnesium sulfate. The desiccant was filtered off and the solvent was evaporated to obtain a white solid, which was then added to 10% dilute hydrochloric acid solut...
Embodiment 3
[0070] Example 3: PTPVS-C 12 preparation of
[0071] Polymer PVTVPS-C 12 The structure is as III, and its synthetic method is referred to (1)~(6) of example 1, and the difference with example 1 is that the reaction monomer adopted in the (4) step of example 1 is 4-bromomethyl-4'- dodecyloxybiphenyl, and this example uses 4-bromomethyl-4'-dodecyloxystyrylbenzene, and the subsequent steps are changed accordingly.
[0072] The product was a dark green solid in 88% yield. 1 H NMR (CDCl 3 , 400MHz, δ-ppm): 7.40 (br, 10H); 7.17 (br, 4H); 7.04 (br, 2H); 6.99-6.87 (br, 11H); 6.90 (br, 2H); 3.92 (br, 4H ); 1.77 (br, 4H); 1.42 (br, 4H); 1.26 (br, 54H); 0.87 (s, 9H). 13 C NMR (CDCl 3 , 100MHz, δ-ppm): 158.71, 139.98, 138.21, 135.96, 132.74, 127.96, 126.98, 125.05, 114.75, 68.07, 50.16, 31.93, 30.83, 29.65, 29.44, 29.31, 28.595, 26.09 13.61.
[0073]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com