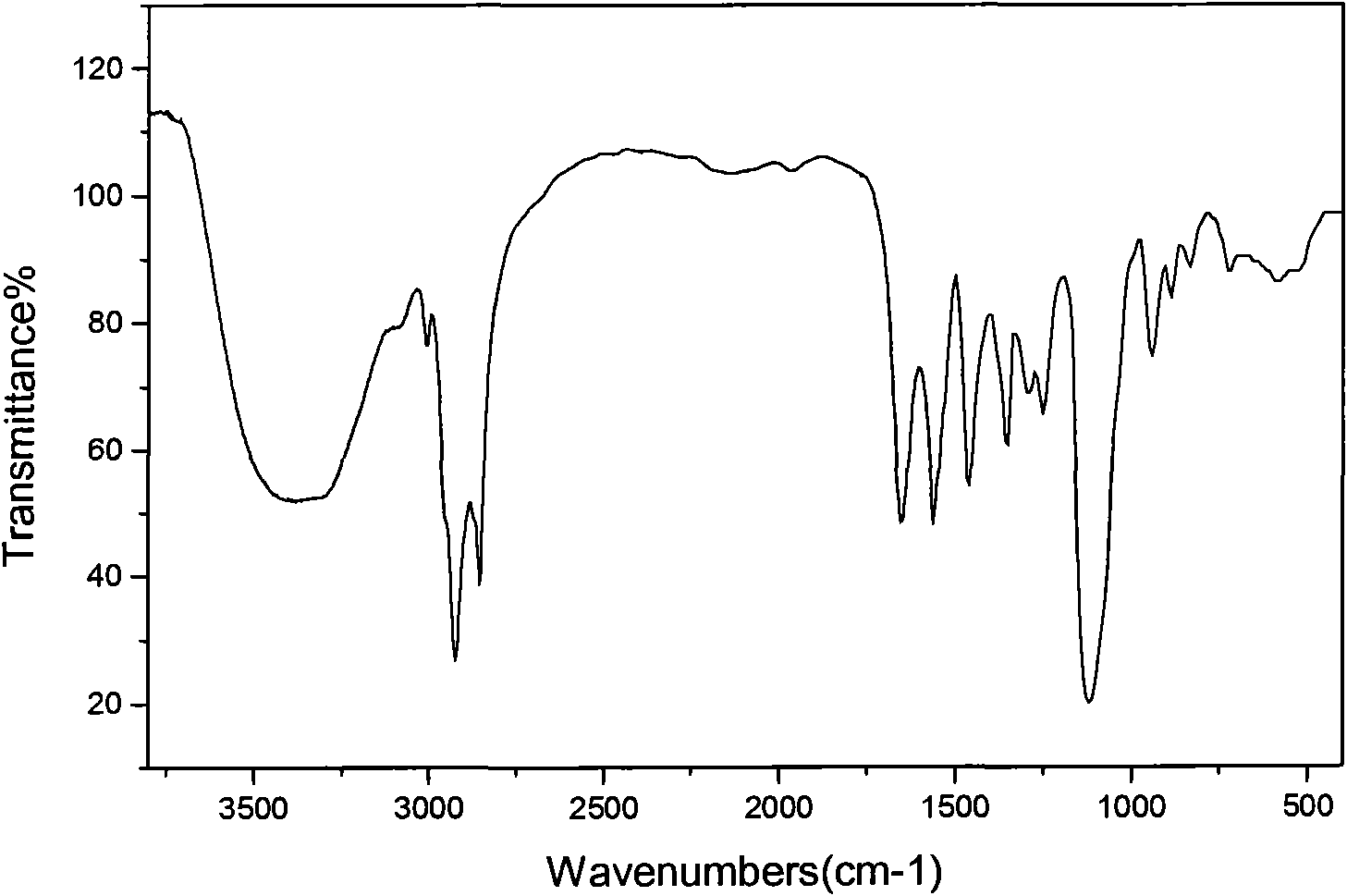
High efficiency anti-magnesium ions oil displacement agent and its preparation method
The technology of magnesium ion and oil displacement agent is applied in the field of high-efficiency anti-magnesium ion oil displacement agent and its preparation. Strong ability, excellent performance, high salinity resistance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
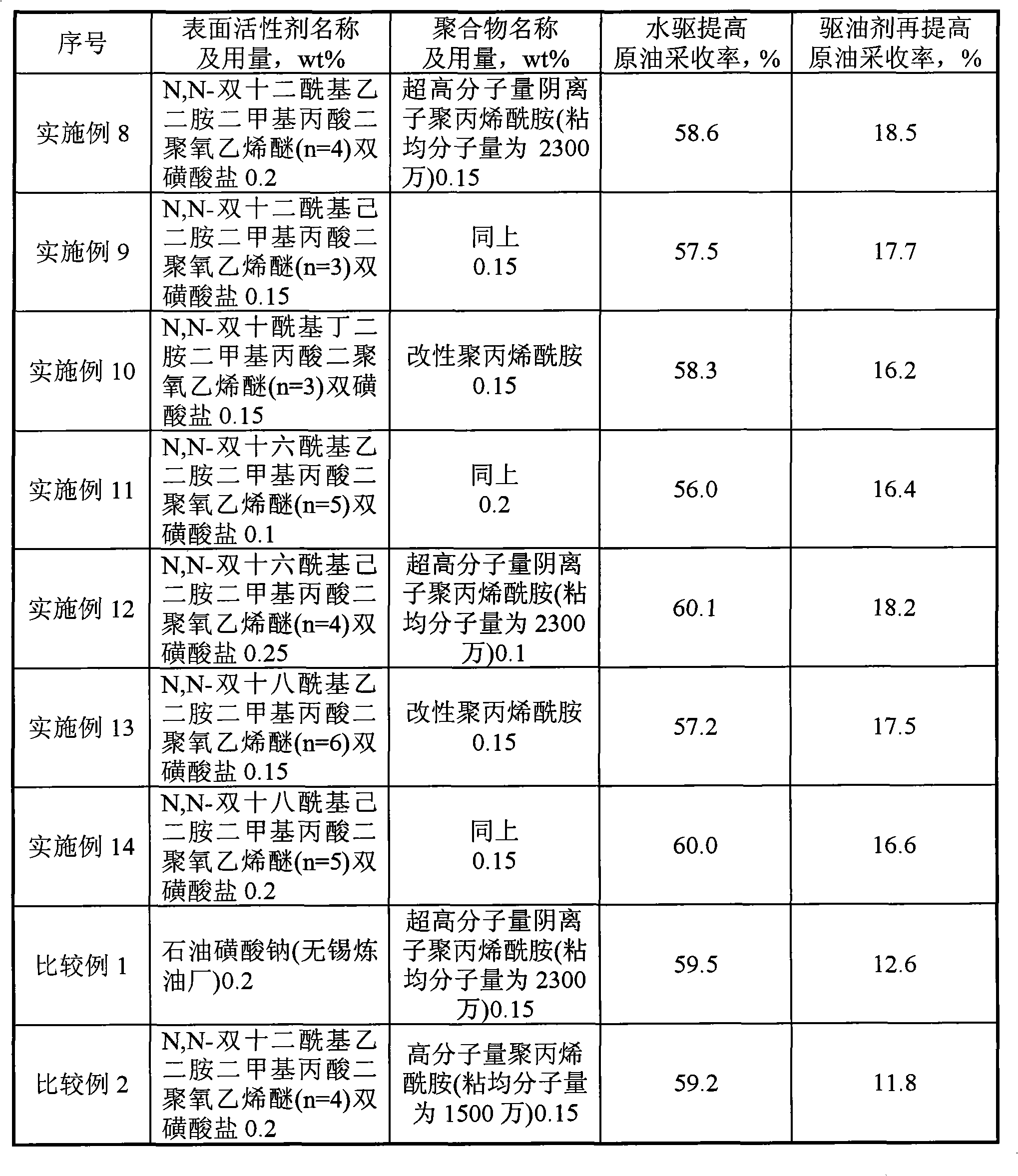
Examples
Embodiment 1
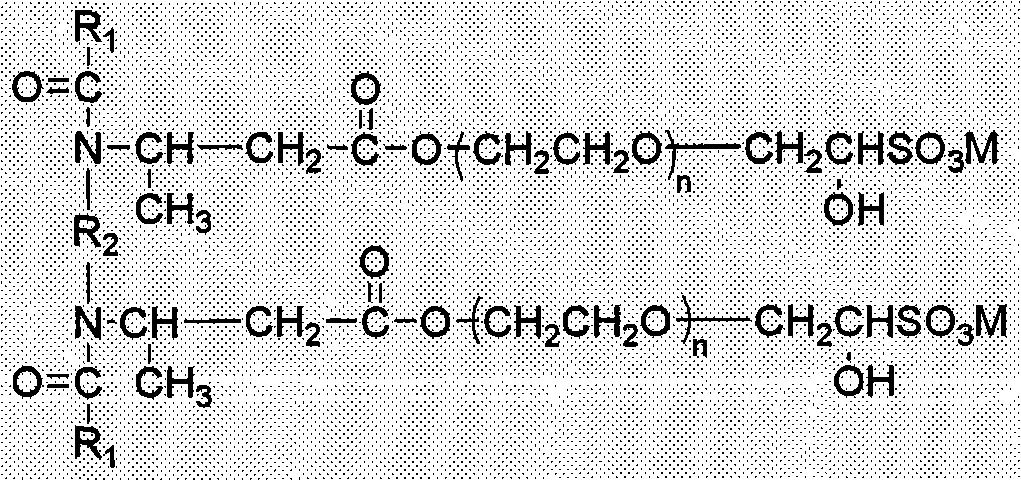
[0033] (a) Synthesis of N, N-dilauroyl ethylenediamine dimethyl propionic acid
[0034] 300 grams (1.5 moles) of lauric acid, 267.8 grams (2.25 moles) of thionyl chloride and 4.5 grams of DMF were added to a 1000-milliliter four-necked flask equipped with a sealed mechanical stirrer, a thermometer, a condenser tube, etc., and reacted at 90 ° C for 3 After one hour, excess thionyl chloride was distilled off under reduced pressure to obtain lauroyl chloride. Add 45.0 grams (0.75 moles) of anhydrous ethylenediamine and 180 grams of 1,4-dioxane to another 2000 ml four-neck flask equipped with a sealed mechanical stirrer, a thermometer, and a condenser tube, heat up to 50° C., and slowly Add 165.0 grams (1.65 moles) of methyl methacrylate dropwise, continue the reaction for 3 hours after dropping, remove unreacted methyl methacrylate and solvent 1,4-dioxane under reduced pressure, and the residue is ethylenediamine dioxane. Methyl methpropionate. Use 50wt% sodium hydroxide to adj...
Embodiment 2
[0041] (a) Synthesis of N, N-dilauroyl hexamethylenediamine dimethylpropionic acid
[0042] With [Example 1] (a), the difference replaces 45.0 grams (0.75 moles) of anhydrous ethylenediamine with 87.0 grams (0.75 moles) of anhydrous hexamethylenediamine, and the rest are the same to obtain N, N-dilauroyl Hexamethylenediamine dimethyl propionic acid 328.6 grams, molar yield 67.2%.
[0043] (b) Synthesis of N,N-dilauroylhexamethylene diamine dimethyl propionate dipolyoxyethylene ether (n=3)
[0044] With [Example 1] (b), the difference is 328.6 grams (0.504 moles) of N, N-dilauroyl hexamethylenediamine dimethyl propionic acid instead of 306.2 grams (0.514 moles) of N, N-didodecanoic acid The consumption of acyl ethylenediamine dimethyl propionic acid and oxirane is 133.1g (3.024 moles), and the consumption of the basic compound of calcium is 7.5 grams to obtain N, N-dilauroyl hexamethylene diamine Dipolyoxyethylene methpropionate (n=3) was 409.5 grams, and the molar yield was ...
Embodiment 3
[0049] (a) Synthesis of N, N-didecanoyl butanediamine dimethylpropionic acid
[0050] With [Example 1] (a), the difference replaces 300.0 grams (1.5 moles) of lauric acid with 261.0 grams (1.5 moles) of capric acid, and replaces 45.0 grams (0.75 grams) of anhydrous butanediamine with 66.0 grams (0.75 moles). mol) anhydrous ethylenediamine, and the rest are the same to obtain 283.3 grams of N, N-didecanoyl butanediamine dimethylpropionic acid, and the molar yield is 66.5%.
[0051] (b) Synthesis of N, N-didecanoyl butanediamine dimethyl propionate dipolyoxyethylene ether (n=3)
[0052] With [Example 1] (b), the difference is 283.3 grams (0.499 moles) of N, N-didecanoyl butanediamine dimethyl propionic acid instead of 306.2 grams (0.514 moles) of N, N-dilauroyl The consumption of ethylenediamine dimethylpropionic acid and oxirane is 131.7g (2.994 moles), and the consumption of the basic compound of calcium is 6.4 grams to obtain N, N-didecanoyl butanediamine dimethyl Dipolyoxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com