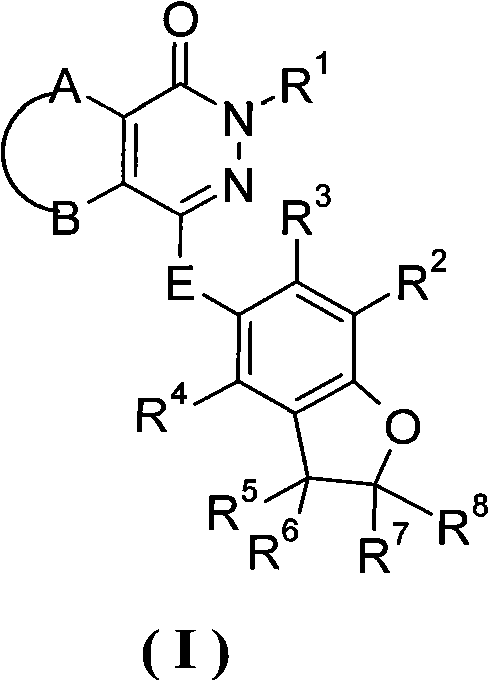
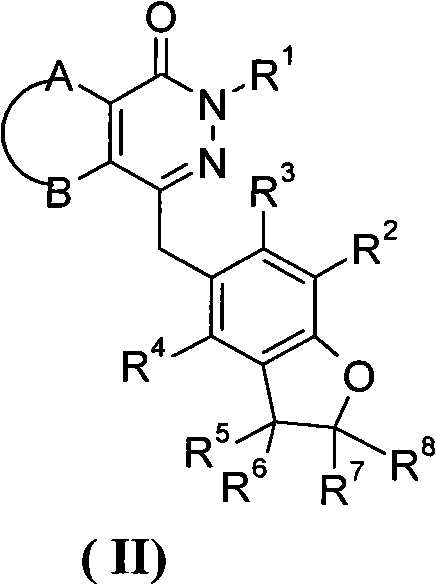
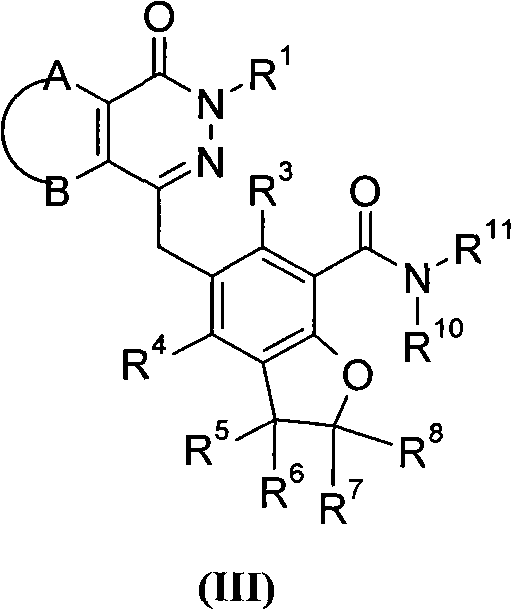
Phthalazinone derivative and its preparation method and use in medicine
A compound, heterocyclic group technology, applied in the field of polymerase inhibitors, can solve problems such as inability to perform repair process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] 4-[(7-bromo-2,3-dihydrobenzofuran-5-yl)methyl]-2H-phthalazin-1-one
[0191]
[0192] first step
[0193] 2,3-Dihydrobenzofuran-5-carbaldehyde
[0194] 2,3-Dihydrobenzofuran 1a (2g, 16mmol) was dissolved in N,N-dimethylformamide (2.60g, 35.20mmol) under stirring, and phosphorus oxychloride (5.20 g, 52mmol), reacted at 90°C for 7 hours, cooled to room temperature, poured into 20mL of ice water, and stirred for 12 hours. Extract with toluene (15mL×3), combine the organic phases, wash with saturated sodium bicarbonate solution (20mL) and saturated sodium chloride solution (20mL) successively, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and use a silica gel column The resulting residue was purified by chromatography with eluent system B to give the title product 2,3-dihydrobenzofuran-5-carbaldehyde 1b (1.70 g, colorless oil), yield: 70.8%.
[0195] MS m / z(ESI): 149.1[M+1]
[0196] second step
[0197] 7-bromo-2,3-dihydr...
Embodiment 2
[0210] 4-(2,3-Dihydrobenzofuran-5-ylmethyl)-2H-phthalazin-1-one
[0211]
[0212]Dissolve 4-[(7-bromo-2,3-dihydrobenzofuran-5-yl)methyl]-2H-phthalazin-1-one 1 (177mg, 0.50mmol) in 5mL tetrahydrofuran with stirring, Tetramethylethylenediamine (116mg, 1mmol) was added, cooled to -78°C, 0.6mL of 1.6M n-butyl lithium in n-hexane was added dropwise, and kept at -78°C for 3 hours. Add 5 mL of water, extract with ethyl acetate (15 mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title product 4-(2,3-dihydrobenzofuran-5 -ylmethyl)-2H-phthalazin-1-one 2 (85 mg, white solid), yield: 61.6%.
[0213] MS m / z(ESI): 279.1[M+1]
[0214] 1 H NMR (400MHz, CDCl 3 ): δ10.50(s, 1H), 8.46-8.48(m, 1H), 7.74-7.80(m, 3H), 7.04-7.10(m, 2H), 6.71-6.73(m, 1H), 4.54(t , 2H), 4.23(s, 2H), 3.16(t, 2H)
Embodiment 3
[0216] 4-[[7-(4-methylpiperazine-1-carbonyl)-2,3-dihydrobenzofuran-5-yl]methyl]-2H-phthalazin-1-one
[0217]
[0218] first step
[0219] 5-[(4-Oxo-3H-phthalazin-1-yl)methyl]-2,3-dihydrobenzofuran-7-carboxylic acid methyl ester
[0220] 4-[(7-bromo-2,3-dihydrobenzofuran-5-yl)methyl]-2H-phthalazin-1-one 1 (480mg, 1.34mmol), palladium acetate (30mg, 0.13mmol ) and 1,3-bis(diphenylphosphine)propane (55mg, 0.13mmol) were placed in a dry reaction flask, sealed and passed into carbon monoxide, and 10mL containing triethylamine (0.41g, 4mmol) and methanol (0.13 g, 4 mmol) of dimethyl sulfoxide, reacted at 75°C for 24 hours. Add 15 mL of water, extract with dichloromethane (20 mL×5), combine the organic phases, wash with water (30 mL) and saturated sodium chloride solution (30 mL×2) successively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. The title product 5-[(4-oxo-3H-phthalazin-1-yl)methyl]-2,3-dihydrobenzofuran-7-carboxylic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com