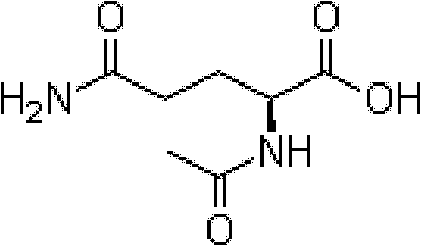
More stable aceglutamide compound and medicinal composition thereof
A technology of acetylglutamine and compounds, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of greater than 0.2%, and achieve the effects of long storage period, low impurity content, and good crystal shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
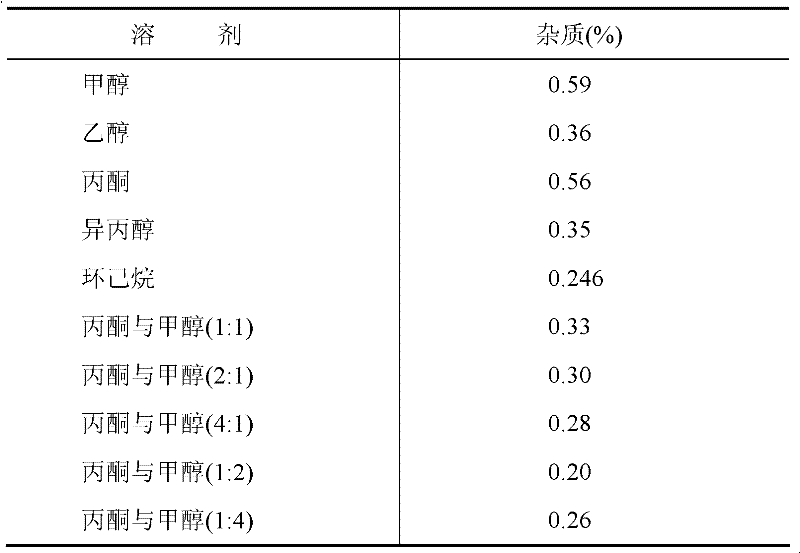
[0037] Add 45g of the purchased crude product acetylglutamine to 450ml of a mixed solution of methanol and acetone with a volume ratio of 2:1, then add 22g of activated carbon, heat to reflux for 2 hours, cool and place in a 4-degree refrigerator for 12 hours to precipitate crystals, and filter Crystals were obtained, and the above recrystallization steps were repeated to obtain 40 g of white crystals with a purity of 99.80%. Each impurity content is less than 0.1%.
Embodiment 2
[0039]
[0040] Its preparation method is as follows:
[0041] Take 100g of acetylglutamine, mix and grind it with PEG-400, dissolve the ground product of acetylglutamine and PEG-400 with 8000ml of absolute ethanol, add 0.5% activated carbon, heat to 80°C and maintain it for 15 minutes, filter to remove carbon, and then Add sodium bisulfite and edetate disodium, stir to dissolve, add absolute ethanol to 10000ml, add 0.5% activated carbon, boil, fine filter with 0.45μm microporous membrane, and use 0.20μm microporous filter The membrane is sterilized and filtered, canned, sterilized, and labeled, and the product is obtained.
Embodiment 3
[0043]
[0044] Its preparation method is as follows:
[0045] Take 60g of acetylglutamine, mix and grind it with PEG-400, dissolve the ground product of acetylglutamine and PEG-400 with 8000ml of absolute ethanol, add 0.5% activated carbon, heat to 80°C and maintain it for 15 minutes, filter to remove carbon, and then Add sodium bisulfite and edetate disodium, stir to dissolve, add absolute ethanol to 10000ml, add 0.5% activated carbon, boil, fine filter with 0.45μm microporous membrane, and use 0.20μm microporous filter The membrane is sterilized and filtered, canned, semi-sealed, placed in a freeze-drying box, freeze-dried, sealed, labeled, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com