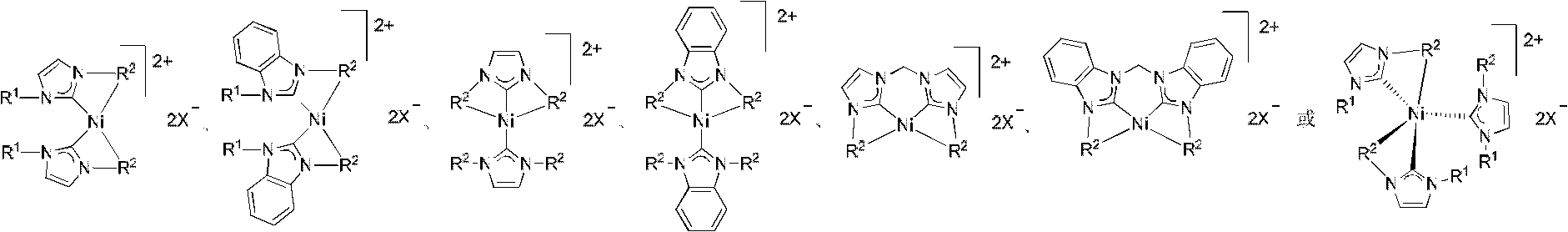
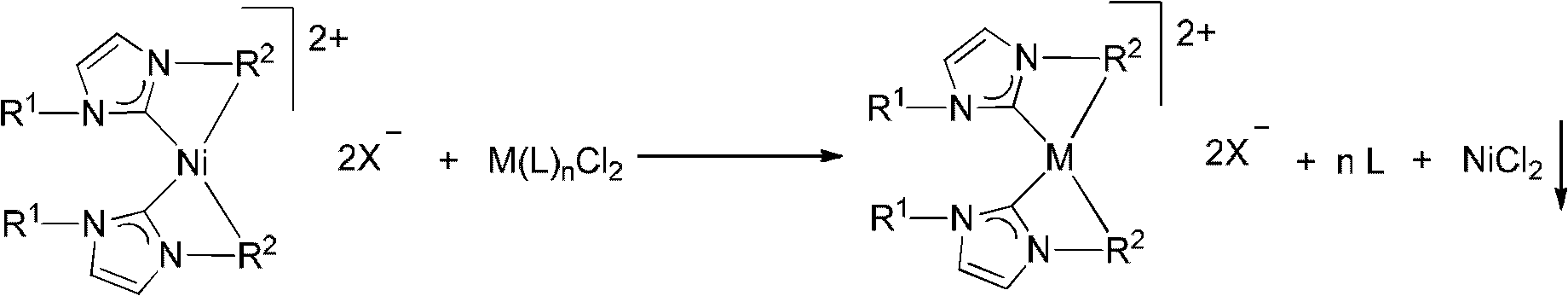
Method for synthesizing metal N-heterocyclic carbene complex
A technology for nitrogen heterocyclic carbene and heterocyclic carbene, which is applied in the field of synthesizing metal nitrogen heterocyclic carbene complexes, can solve the problems of difficulty in preparation and storage, unstable free carbene, easy self-polymerization, etc., and achieves high yield and cost. Inexpensive, well-tolerated results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
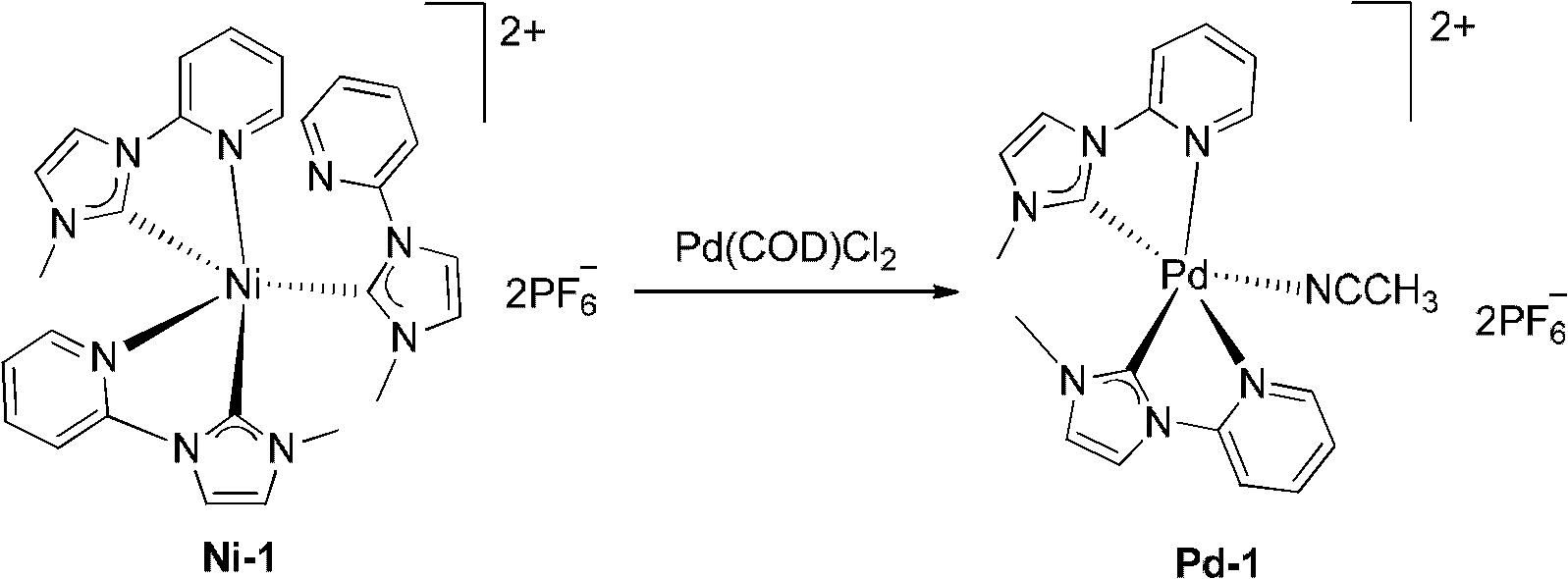
[0039] Example 1 complex Pd-1 (C 20 h 21 f 12 N 7 P 2 Preparation of Pd)
[0040]
[0041] In a 50mL flask, add 826mg (1mmol) nickel nitrogen heterocyclic carbene complex Ni-1, 10mL acetonitrile solvent, 286mg (1mmol) (1,5-cyclooctadiene) palladium dichloride (Pd(COD)Cl 2 ), stirred and reacted for 8 hours in an oil bath at 60-70°C, filtered through a 300-mesh silica gel layer to obtain a colorless solution, concentrated to 3mL, added 15mL of diethyl ether to crystallize and the white powder obtained was the palladium nitrogen heterocyclic carbene complex Pd- 1. Purified by recrystallization to obtain 480 mg of colorless crystals, with a yield of 63%. 1 H NMR (400MHz, DMSO-d 6 ): δ8.97(d, J=4.8Hz, 1H, PyH), 8.58(br, 1H, PyH), 8.56(s, 1H, imidazolylidene H), 8.42(t, J=7.6Hz, 1H, PyH) , 8.40 (s, 1H, imidazolylidene H), 8.19 (d, J=8.0Hz, 1H, PyH), 8.18 (s, 1H, imidazolylidene H), 8.07 (t, J=7.8Hz, 1H, PyH), 7.88 (s, 1H, PyH), 7.73(t, J=6.0Hz, 1H, PyH), 7.57(s, 1H, imid...
Embodiment 2
[0042] Embodiment 2 complex Pd-2 (C 19 h 18 CIF 6 N 4 PPd) Preparation
[0043]
[0044] In a 50mL flask, add 953mg (1mmol) nickel nitrogen heterocyclic carbene complex Ni-2, 10mL acetonitrile, 571mg (2mmol) (1,5-cyclooctadiene) palladium dichloride (Pd(COD)Cl 2 ), in an oil bath at 50-60°C, stirred and reacted for 12 hours, filtered through a 300-mesh silica gel layer to obtain a yellow solution, concentrated to 3 mL, added 15 mL of ether to crystallize to obtain a yellow powder, which is the palladium nitrogen heterocyclic carbene complex Pd-2, Purified by recrystallization to obtain 1080 mg of yellow crystals with a yield of 92%. 1 H NMR (400MHz, DMSO-d 6 ): δ9.05(d, J=8.8Hz, 1H), 8.83(d, J=8.4Hz, 1H), 8.54(s, 2H, NCHCHN+phenanthroline H), 8.39(d, J=8.8Hz, 1H ), 8.14(s, 2H), 8.06(dd, J=8.0, J=5.2Hz, 1H), 7.82(d, J=2Hz, 1H, NCHCHN), 4.36(t, J=6.8Hz, 2H, NCHN 2 CH 2 CH 2 CH 3 ), 1.78 (m, 2H, NCH 2 CH 2 CH 2 CH 3 ), 1.33 (m, 2H, NCH 2 CH 2 CH 2 CH 3 ), 0.9...
Embodiment 3
[0045] Example 3 Complex Pd-3(C 20 h 22 f 12 N 6 P 2 Preparation of Pd)
[0046]
[0047] In a 50mL flask, add 695mg (1mmol) nickel nitrogen heterocyclic carbene complex Ni-3, 10mL acetonitrile, 286mg (1mmol) (1,5-cyclooctadiene) palladium dichloride (Pd(COD)Cl 2 ), stirred and reacted for 16 hours in an oil bath at 60-70°C, filtered through a 300-mesh silica gel layer to obtain a colorless solution, concentrated to 3 mL, added 15 mL of diethyl ether to crystallize to obtain a white powder, which is the palladium nitrogen heterocyclic carbene complex Pd-3 , and purified by recrystallization to obtain 640 mg of colorless crystals with a yield of 86%. 1 H NMR (400MHz, DMSO-d 6 ): δ8.29 (d, J=5.2Hz, 2H, 6-PyH), 8.24 (t, J=7.6Hz, 2H, 4-PyH), 7.96 (d, J=7.6Hz, 2H, 3-PyH ), 7.76(d, J=1.6Hz, 2H, NCHCHN), 7.62(t, J=6.4Hz, 2H, 5-PyH), 7.49(d, J=1.6Hz, 2H, NCHCHN), 6.17(d, J=14.8Hz, 2H, NCH 2 ), 5.80 (d, J=15.6Hz, 2H, NCH 2 ), 3.31 (s, 6H, NCH 3 ). 13 CNMR (100MHz, DMSO-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com