Endotoxin adsorption medium based on magnetic chitosan microballoon and its application method
A technology of chitosan microspheres and adsorption media, applied in the field of biomedical materials, can solve the problems of small processing capacity and large loss of active ingredients, and achieve high selectivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of chitosan-histidine endotoxin adsorption medium (MCM-His)
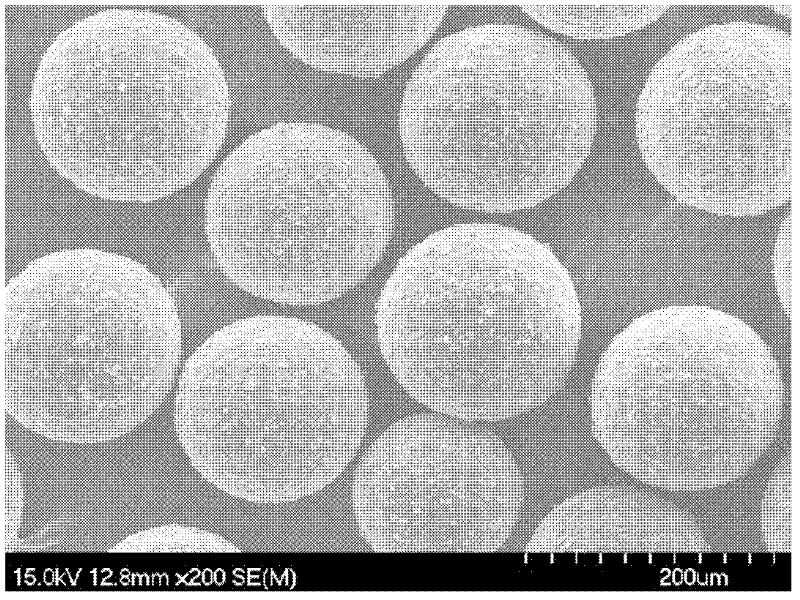
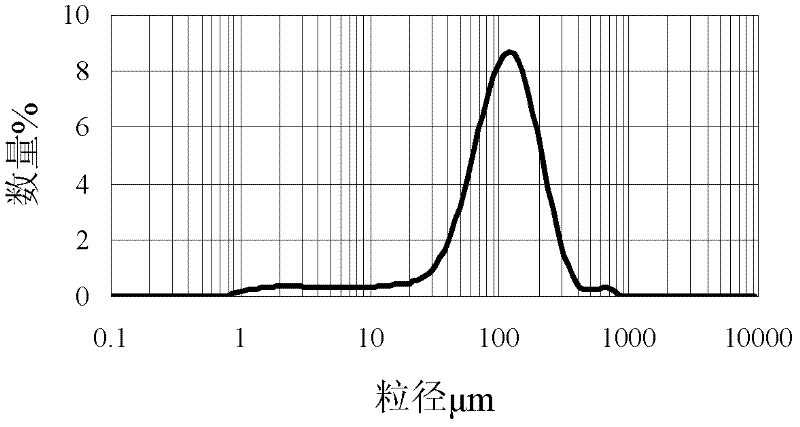
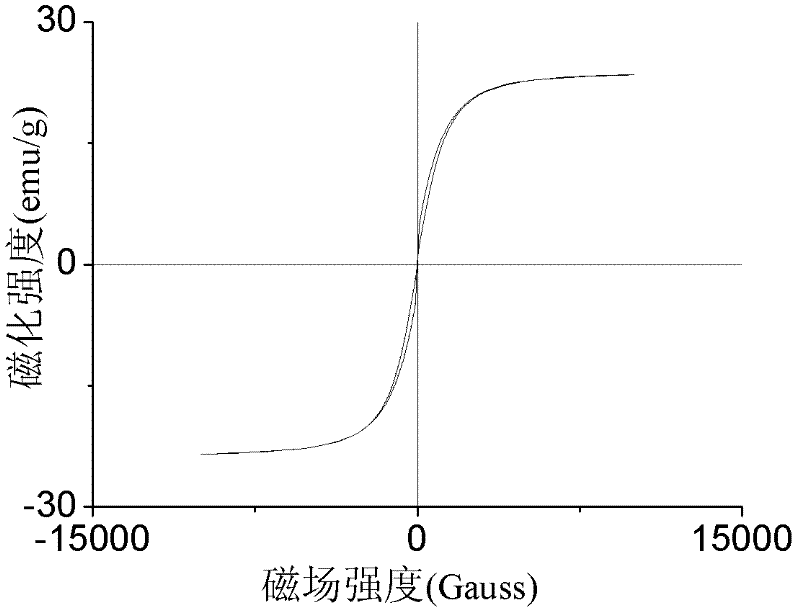
[0025] (1) Ultrasonic dispersion of 1.5g ferroferric oxide in 100mL of acetic acid solution with a volume fraction of 2%, adding 3g chitosan (produced by Aoxing Company, molecular weight 300,000, deacetylation degree 95%, i.e. amino density is 5833 μ mol / g) was completely dissolved and dispersed into 100 mL of liquid paraffin containing 1 mL of Span 80, stirred for 30 min, and then added dropwise with 3 mL of glutaraldehyde solution with a volume fraction of 25%, reacted at 40°C for 2 h, and used a mass fraction of 10% Adjust the pH of the NaOH solution to about 12, and at the same time raise the temperature to 70°C, continue to stir and react for 2h, the product is washed with petroleum ether and hot ethanol, and then vacuum-dried to obtain magnetic chitosan microspheres (MCM), which are determined by acid-base titration. Amino density: Disperse 50mg of microspheres into 20ml o...
Embodiment 2
[0028] Embodiment 2: the preparation of chitosan-tetraethylenepentamine endotoxin adsorption medium (MCM-TEPA)
[0029] (1) Ultrasonic dispersion of 2.0g ferric ferric oxide in 100mL of 5% acetic acid solution by volume fraction, adding 4g chitosan (produced by Aoxing Company, molecular weight 300,000, deacetylation degree 95%, i.e. amino density is 5833 μ mol / g) was completely dissolved and then dispersed into 200mL of liquid paraffin containing 3mL of Span 80. After stirring for 30min, 4mL of glutaraldehyde solution with a volume fraction of 25% was added dropwise and reacted at 40°C for 1h. Adjust the pH of the NaOH solution to about 12, and at the same time raise the temperature to 70°C, continue to stir and react for 3 hours, wash the product with petroleum ether and hot ethanol, and then dry it in vacuum to obtain magnetic chitosan microspheres.
[0030] (2) Disperse 0.5 g of magnetic chitosan microspheres obtained in step (1) into a mixed solution of 10 mL dimethyl sul...
Embodiment 3
[0034] Example 3: Use of Adsorption Media
[0035] Get respectively magnetic chitosan microspheres (the MCM that embodiment 1 makes), magnetic chitosan microspheres-histidine (MCM-His that embodiment 1 makes) and magnetic chitosan microspheres-tetraethylenepenta Amine (MCM-TEPA prepared in Example 2) 50 mg was washed with 1% sodium deoxycholate aqueous solution for three times by suction filtration or magnetic field separation, then washed three times with pyrogen-free water, dried and dispersed into 2 mL of endotoxin-containing solution (tap water), shake and adsorb for 30 minutes, absorb the adsorption medium with a magnet, and take out the supernatant to measure the endotoxin content.
[0036]The endotoxin content was determined by the limulus reagent chromogenic matrix method, and the adsorption kinetic curve of magnetic chitosan microspheres-histidine (MCM-His) to endotoxin was as follows: Figure 4 The adsorption effects of different adsorption media are shown as Figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com