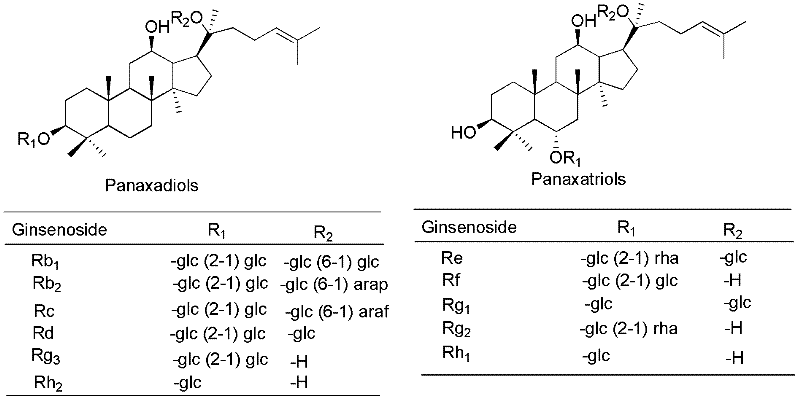
Synthesis method for 20-bit sugar connected protopanaxatriol analog ginsenoside and analog
A technology of protopanaxatriol and its synthesis method, which is applied in the field of preparation of protopanaxatriol ginsenoside F1 and its analogues, and can solve the problems of difficult acquisition, high price of enzymes, difficulty in providing ginsenoside samples, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Selective Protection of Protopanaxatriol 12-OH Using Methyl
[0047]
[0048] Reagents and conditions: a) MeI (1.2 equiv.), LiHMDS (1.2 equiv.), THF, 0°C. Wherein LiHMDS is lithium hexamethyldisilazide; THF is tetrahydrofuran; the equivalents of the reagents used are relative to the original ginseng three Alcohol equivalent.
[0049] Specific experimental process and data:
[0050] Dissolve protopanaxatriol (477mg, 1.0mmol) in dry THF (5mL), cool the reaction system to 0°C and slowly add LiHMDS (1.2mmol) dropwise to the system, stir at 0°C for 10 minutes and then add MeI (1.2 mmol), stirring was continued for 2 hours. The reaction was quenched with saturated ammonium chloride, extracted with a large amount of ethyl acetate, washed with water (3*30mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated, followed by column chromatography (petroleum ether: ethyl acetate = 2: 1) Obtain 417 mg (85%) of compound 2a. [α] D 25 =...
Embodiment 2
[0052] Selective protection of protopanaxatriol 12-OH by benzyl group
[0053]
[0054] Reagents and conditions: a) BnBr (1.2equiv.), LDA (1.2equiv.), THF, 0°C. Wherein BnBr is benzyl bromide; LDA is lithium diisopropylamide; THF is tetrahydrofuran; the reagent equivalents used are relative Equivalent to protopanaxatriol.
[0055] Specific experimental process and data:
[0056] Dissolve protopanaxatriol (477mg, 1.0mmol) in dry THF (5mL), cool the reaction system to 0°C, slowly add LDA (1.2mmol) dropwise to the system, stir at 0°C for 10 minutes, then add BnBr (1.2 mmol), stirring was continued for 2 hours. The reaction was quenched with saturated ammonium chloride, extracted with a large amount of ethyl acetate, washed with water (3*30mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated, followed by column chromatography (petroleum ether: ethyl acetate = 2: 1) Obtain 510 mg (90%) of compound 2b. [α] D 25 =+15.0 (c 1.0, CHCl...
Embodiment 3
[0058] Selective Protection of Protopanaxatriol 12-OH Using Piv(pivaloyl) Group
[0059]
[0060] Reagents and conditions: a) PivCl (1.2 equiv.), Et 3 N, DCM, -5°C. Wherein PivCl is pivaloyl chloride; DCM is dichloromethane; the equivalents of the reagents used are equivalents relative to protopanaxatriol.
[0061] Specific experimental process and data:
[0062] Protopanaxatriol (200mg, 0.4mmol) was dissolved in dry DCM (5mL), the reaction system was cooled to -5°C, and dry triethylamine (0.4mL) was slowly added dropwise to the system, then at -5°C PivCl (0.13 Ml, 1.2 equiv.) was added dropwise to the reaction system, and stirring was continued for 1.5 hours. The reaction was quenched with saturated ammonium chloride, extracted with a large amount of ethyl acetate, washed with water (3*30mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated, and column chromatography gave 210 mg (89%) of compound 2c.
[0063] [α] D 25 =+10.2(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com