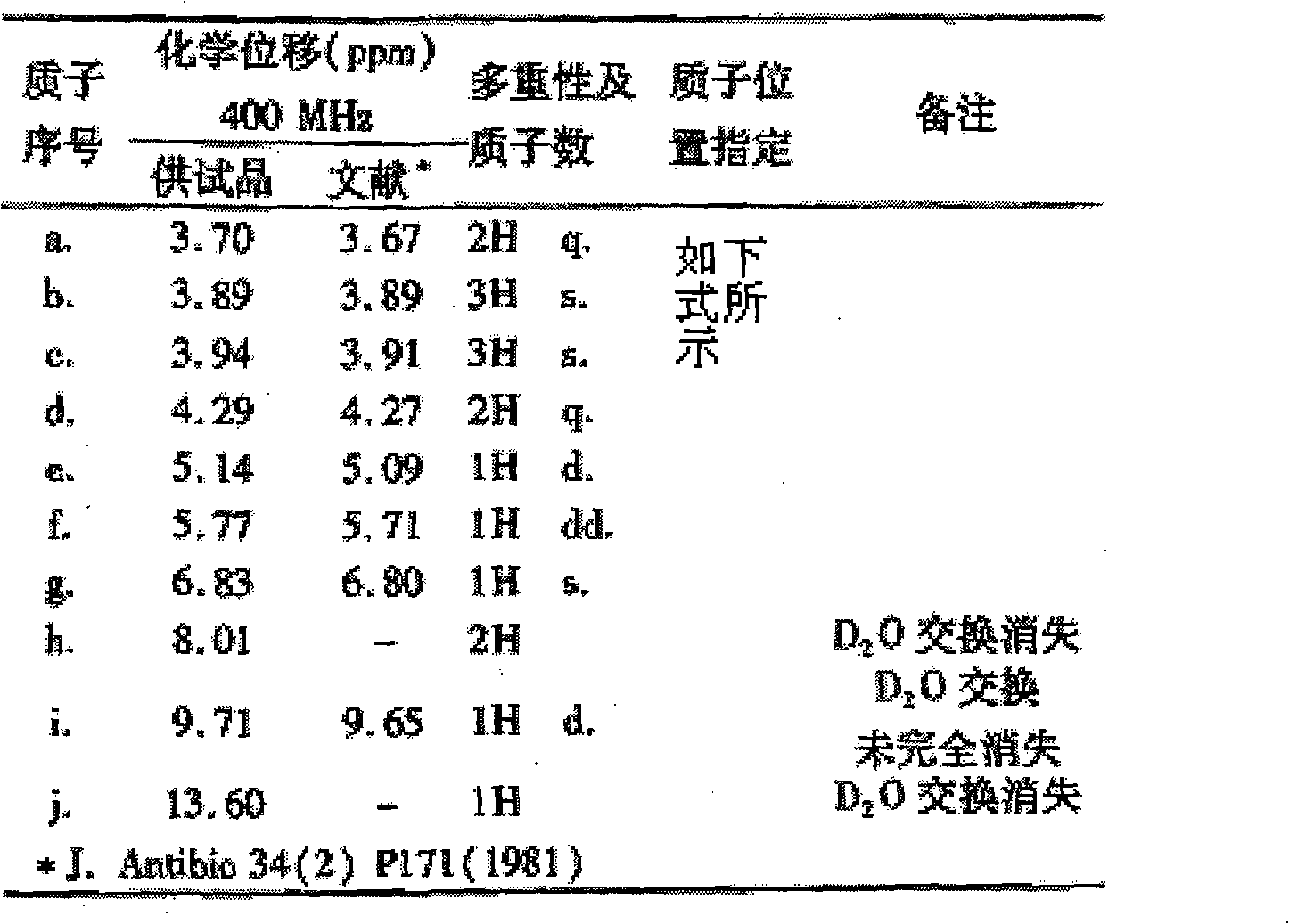
Novel method for preparing cefmenoxime hydrochloride compound
A technology of cefmenoxime hydrochloride and its compound, which is applied in the field of new preparation methods for purifying cefmenoxime hydrochloride, and can solve the problems of low purity of cefmenoxime hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Take 100 g of crude cefmenoxime hydrochloride prepared according to CN101555251A, the content of cefmenoxime hydrochloride as measured by high performance liquid chromatography is 89%, and the content of polymer as determined by gel chromatography system is 4%. Add ethanol to the raw material cefmenoxime hydrochloride, control the temperature not to exceed 28°C, stir vigorously, and then filter, wash the filter cake with ethanol at a temperature not exceeding 20°C, and air dry.
[0061] The filter cake is put into 20% ammonia water, and the treatment time is 2 hours, until the aqueous solution reaches weak alkalinity, preferably with a pH value not exceeding 8.5. Stirring is carried out during the treatment, and the separated precipitate is filtered off. Then the aqueous solution was heated to 40°C to remove the remaining ammonia gas components in the aqueous solution.
[0062] Slowly add hydrochloric acid with a concentration of 2 mol / L to the obtained ammonia solutio...
Embodiment 2
[0071] Get 100g cefmenoxime hydrochloride bulk drug (produced by Zhejiang Jianfeng Pharmaceutical Co., Ltd., batch number 20110203), the purity of cefmenoxime recorded by high performance liquid chromatography is 91%, and the polymer impurity content measured by gel chromatography is 1.5%. Add ethyl acetate to the raw material cefmenoxime hydrochloride, control the temperature not to exceed 25°C, stir vigorously, and then filter, wash the filter cake with ethyl acetate at a temperature not exceeding 16°C, and dry in vacuum.
[0072] The filter cake is put into 23% ammonia water, and the treatment time is 3 hours, until the aqueous solution reaches weak alkalinity, preferably the pH value is not more than 9. Stirring is carried out during the treatment, and the separated precipitate is filtered off. Then the aqueous solution was heated to 45°C to remove the remaining ammonia gas components in the aqueous solution.
[0073] Slowly add hydrochloric acid with a concentration of 3...
Embodiment 3
[0076] Get 100g of cefmenoxime hydrochloride crude drug (produced by Zhejiang Jianfeng Pharmaceutical Co., Ltd., batch number 20080701) with a longer production date, the purity of cefmenoxime recorded by high performance liquid chromatography is 85%, and the determination of macromolecular impurities by gel chromatography Content 9%. Add ethyl acetate to the raw material cefmenoxime hydrochloride, control the temperature not exceeding 25°C, stir vigorously, and then filter, wash the filter cake with ethyl acetate at a temperature not exceeding 15°C, and dry it in vacuum.
[0077] Put the filter cake into ammonia water with a concentration of 20%, and the treatment time is 4 hours, until the aqueous solution reaches weak alkalinity, preferably the pH value is not more than 8. Stirring is carried out during the treatment, and the separated precipitate is filtered off. Then the aqueous solution was heated to 50°C to remove the remaining ammonia gas components in the aqueous sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com