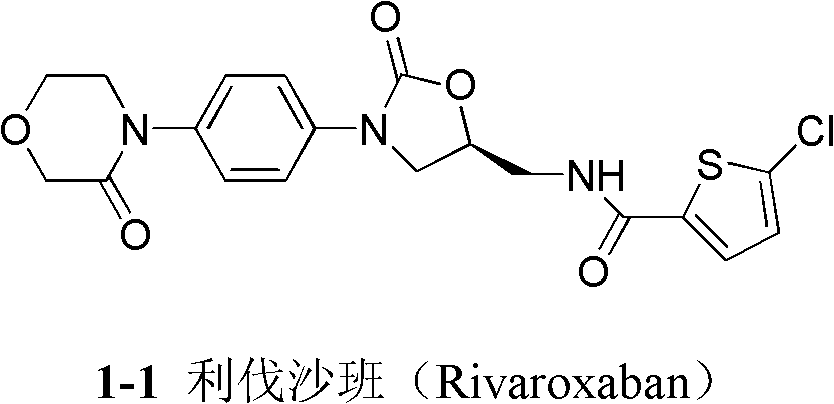
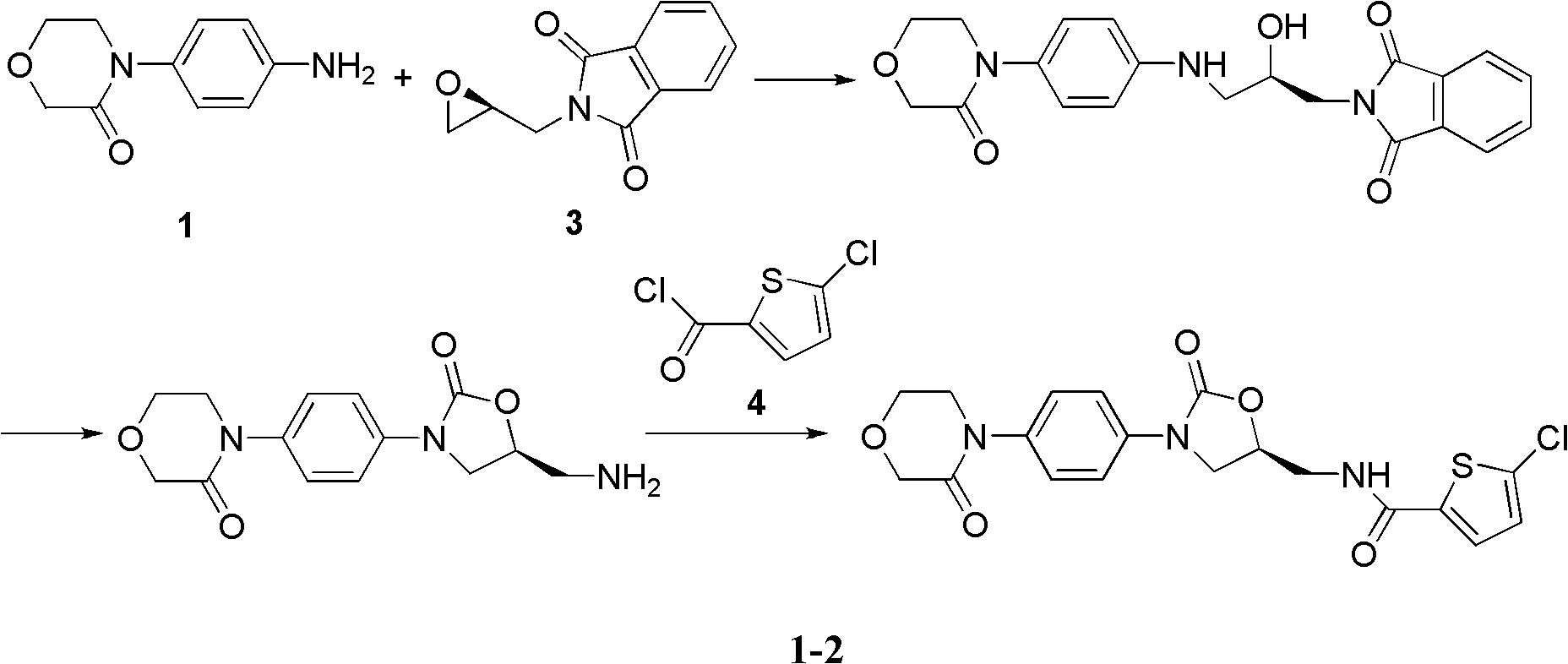
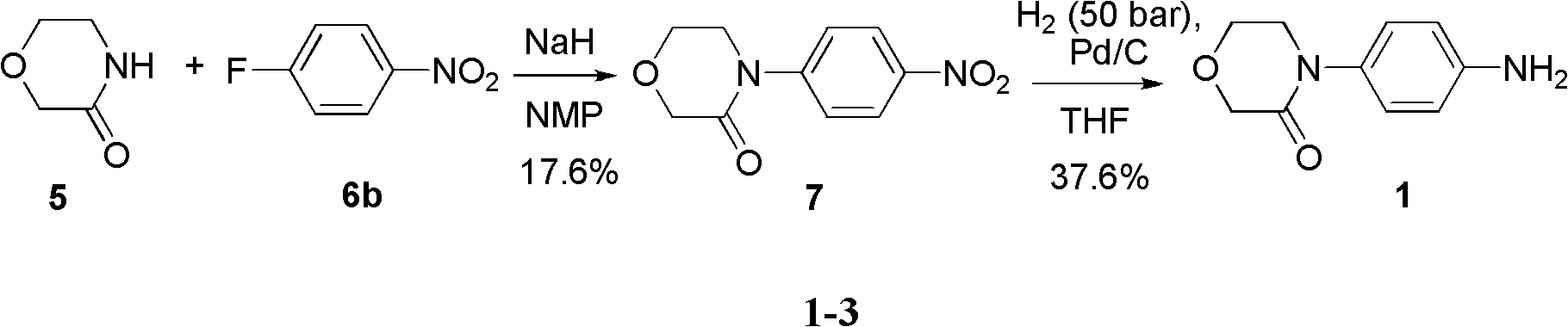
4-(4-amion phenyl)-3-morpholone intermediate amide and synthesis method and application thereof
A technology of aminophenyl and synthetic method, which is applied in the field of drug synthesis, can solve the problems of severe reaction conditions, difficult to obtain raw materials, poor environmental friendliness, etc., and achieve the effect of mild reaction conditions, easy to obtain raw materials, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of 2-(4-nitrophenyl)aminoethanol (10)
[0030] Add 2.02g (0.010mol) p-nitrobromobenzene, 920uL (0.015mol) aminoethanol, and 10mL DMF into a 25mL flask, then add 6.90g (0.05mol) anhydrous potassium carbonate, and heat at 120°C for 24 hours. Solid potassium carbonate was removed by filtration, rinsing with ethyl acetate. The system was spin-dried, and put on the column (silica gel) by dry method, and the P / E (petroleum ether / ethyl acetate) = 1:1 was passed through the column. 229 mg of starting material were recovered. A small amount of column chromatography was used for analysis, and the remaining crude product was directly used for the next reaction.
[0031] 1 H NMR (CDCl 3 , 400MHz): δ8.07(d, 2H), 6.61(d, 2H), 3.88(t, 2H), 3.37(t, 2H).
Embodiment 2
[0032] Example 2 Preparation of 2-(4-aminophenyl)aminoethanol (11)
[0033]Using methanol as a solvent, the compound of Example 1 was hydrogenated overnight at room temperature and pressure with 10% Pd / C. Pd-C was filtered off with celite, spin-dried, and column chromatography (silica gel) yielded 0.778 g of the product. The two-step yield was 59%.
[0034] 1 H NMR (CDCl 3 , 400MHz): δ6.60(d, 2H), 6.54(d, 2H), 3.76(t, 2H), 3.19(t, 2H).
Embodiment 3
[0035] Example 3 Preparation of N-chloroacetyl-N-(4-(N-chloroacetyl)-N-phenyl)aminoethanol (2a)
[0036] Under nitrogen protection, 0.778 g of substrate 11 was dissolved in 5 mL of THF. And add 2.30mL triethylamine to the system and stir at 0°C. Under nitrogen protection, dissolve 0.82mL of chloroacetyl chloride in 5mL of THF, cool to 0°C, and slowly drop into the system. React at 0°C for 4 to 5 hours. The reaction solution was filtered with anhydrous sodium sulfate and rinsed with ethyl acetate. After being spin-dried, it was applied to the column (silica gel) by dry method, P / E (petroleum ether / ethyl acetate) = 4:1 to 2:1, and 539 mg of the product was obtained with a yield of 68%.
[0037] 1 H NMR (CDCl 3 , 400MHz): δ7.67(d, 2H), 7.31(d, 2H), 4.22(s, 2H), 3.90(t, 2H), 3.86(s, 2H), 3.80(t, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com