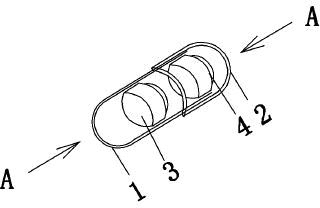
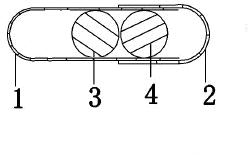
Levalbuterol hydrochloride oral controlled release tablet capsule and preparation method thereof
A levosalbutamol hydrochloride and controlled-release tablet technology, which is applied in the field of medicine, can solve the problems of non-toxicity, long preparation cycle, and complicated process, and achieve the effects of convenient treatment, short preparation cycle, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: the preparation of levosalbutamol hydrochloride pulse tablet
[0085] (1) Preparation of pulsed core
[0086] Levalbuterol Hydrochloride 100g
[0087] Microcrystalline Cellulose KG-801 40g
[0088] Microcrystalline Fiber PH-302 32 g
[0089] Oligomeric Hydroxypropyl Cellulose 40g
[0090] Compressible starch 80g
[0092] Take by weighing main ingredient, microcrystalline cellulose, oligomeric hydroxypropyl cellulose, compressible starch, magnesium stearate and mix uniformly by prescription quantity, compress tablet, obtain the tablet core of 10mg / tablet.
[0093] (2) Preparation of pulse-coated tablets
[0094] Coat the tablet core with 160g of acrylic resin RS, 100g of acrylic resin L100, 10g of triethyl citrate, 30g of talcum powder, and 1000ml of absolute ethanol in a coating pan.
Embodiment 2
[0095] Embodiment 2: Preparation of Levosalbutamol Hydrochloride Sustained-release Tablets
[0096] (1) Preparation of sustained-release tablet cores
[0097] Levalbuterol Hydrochloride 100g
[0098] Lactose 100g
[0099] Microcrystalline Fiber PH-302 30g
[0100] Oligomeric Hydroxypropyl Cellulose 45g
[0101] Polyvinylpyrrolidone (K30) 15g
[0104] 75% ethanol appropriate amount
[0105] Dissolve polyvinylpyrrolidone in an appropriate amount of 75% ethanol, add the prescribed amount of drugs, lactose, microcrystalline fiber PH-302, and soft materials made of oligomeric hydroxypropyl cellulose, pass through a 40-mesh sieve, and ventilate and dry at 60°C. Sieve through a 60-mesh sieve for granulation, add an appropriate amount of magnesium stearate, mix evenly, and press into tablets to obtain a tablet core of 10 mg / tablet.
[0106] (2) Preparation of sustained-release coated tablets
[0107] Coat the tablet core wi...
Embodiment 3
[0108] Example 3: Capsule filling
[0109] Take No. 0 empty gelatin capsules, place them in a capsule filling machine, fill the above-mentioned pulse tablets and sustained-release tablets in each hollow capsule in turn, fill each capsule with one tablet with different drug-release properties, and pack it to obtain this product. Invention of tablet capsules.
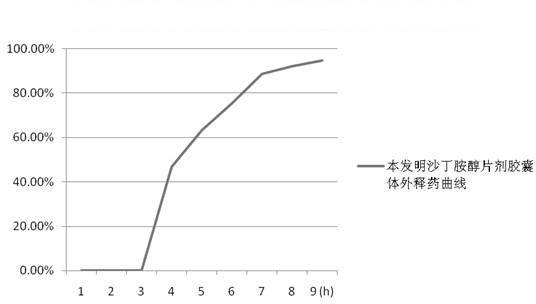
[0110] time (h) 1 2 3 4 5 6 7 8 9 Drug release (%) 0 0 0 46.8 63.1 75.3 88.5 92.1 94.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com