A method to synthesize α-galactoside epitopes on the surface of tumor cells and activate immune cells
A technology of tumor cells and galactoside, which is applied in the direction of tumor/cancer cells, animal cells, vertebrate cells, etc., and can solve problems such as the inability of immunotherapy technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1. Isolation of Tumor Cells from Formalin-Fixed Human Tumor Tissue
[0016] The tumor specimens were cut into tissue pieces smaller than 1cm, soaked in a large amount of cold saline, and shaken on the shaker in the cold storage for at least 6 hours, during which the saline was changed at least three times. Carefully remove all connective tissue around the tumor tissue under a microscope, free tumor cells with a thin curved needle, filter with a cell sieve, remove large cell clumps and connective tissue, and wash the free tumor cells three times with cold saline.
Embodiment 2
[0017] Example 2. Isolation of tumor cells from paraffin-embedded tumor tissue
[0018] Use a scalpel to remove all the paraffin around the tumor specimen as much as possible, prepare a series of Histo-clear solutions, 3 containers with covers, each 100ml, and place them on a shaker in a fume hood; place the treated tumor specimens on the above-mentioned In one container, after all the paraffin is dissolved, transfer to the second and third containers to further remove the paraffin; transfer the above-mentioned dewaxed specimens to the following four alcohol series containers (100%, 50%, 20 % and normal saline), each on a shaker for more than one hour; free tumor cells with a thin curved needle, filter with a cell sieve, remove large cell clumps and connective tissue, and wash free tumor cells three times with cold normal saline.
Embodiment 3
[0019] Example 3. Synthesis of α-Gal epitopes on the surface of fixed tumor cells
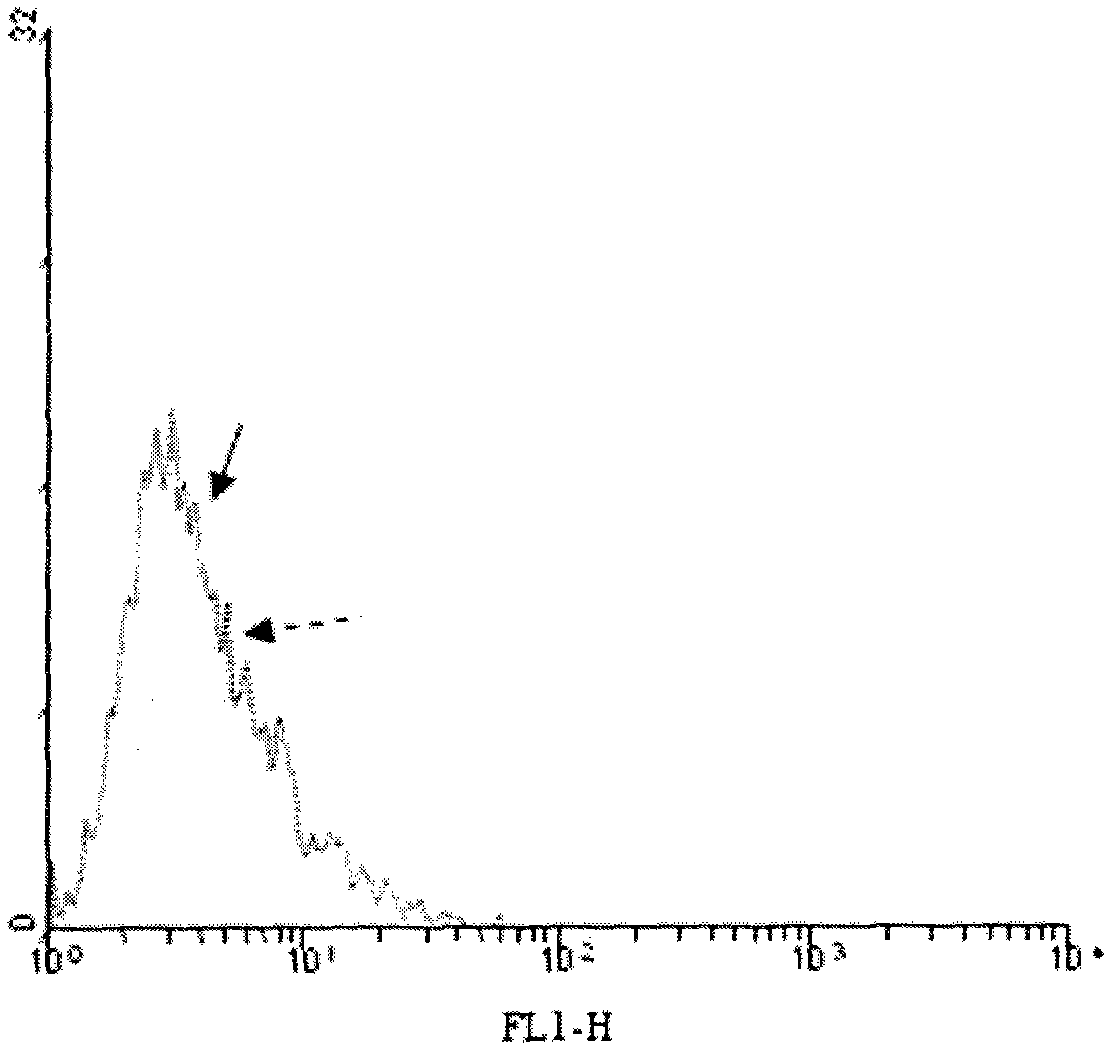
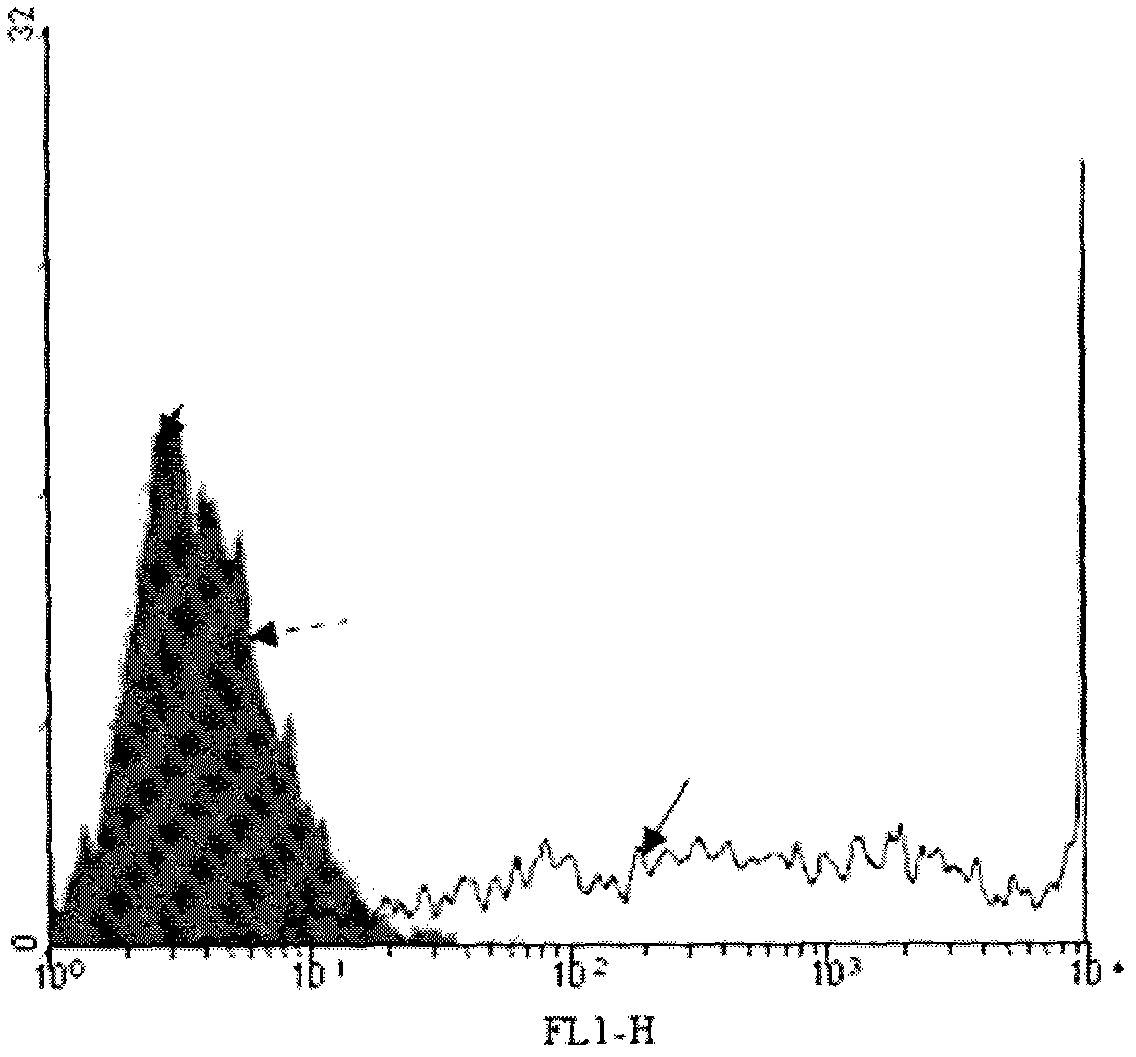
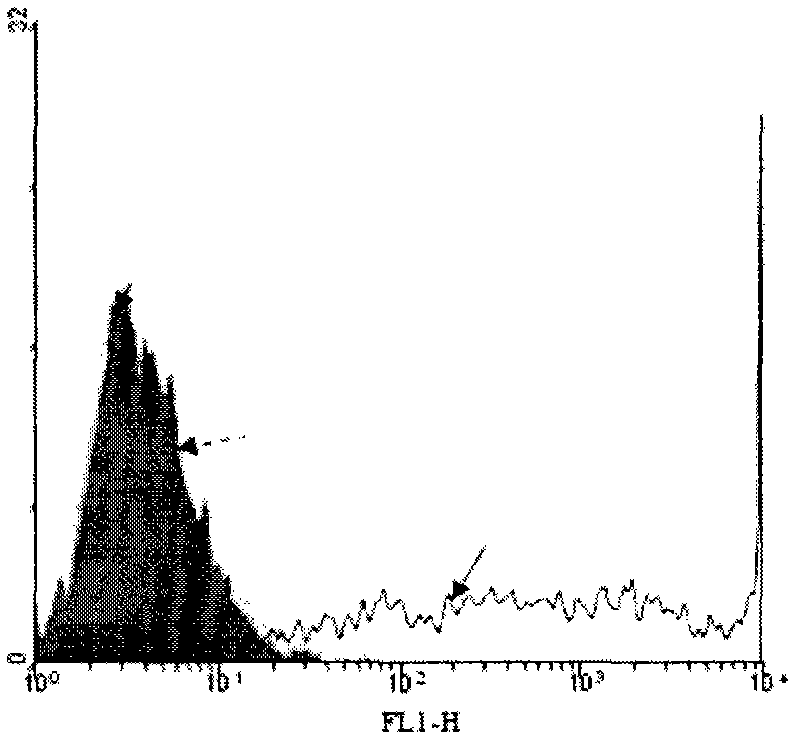
[0020] 3.1 Configure enzyme buffer 1ml (0.1M MES (PH 6.2), 25mM MnCl 2 and 1mM UDP-gal). Resuspend the above isolated tumor cells with 1 ml enzyme buffer. Then add 1mU neuraminidase and 5U recombinant bovine α-1,3-GT to the system, incubate at 37°C for 30 minutes, take out 200μl for flow cytometry:
[0021] Negative control group: no sialidase (neuraminidase), no GT
[0022] Experimental group: with sialidase, with GT
[0023] After the incubation, wash twice with normal saline containing 1 mM EDTA and saline without EDTA respectively (1500 rpm, centrifuged for 10 minutes).
[0024] 3.2 Determination of α-Gal epitope synthesis on the surface of tumor cell membrane by flow cytometry
[0025] Firstly, 1 ml of human serum (containing natural anti-α-Gal epitope antibody) was diluted 1:5 with 1% BSA saline, mixed with cells, and incubated on ice for 60 minutes. Then wash twice with 1% BSA phys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com