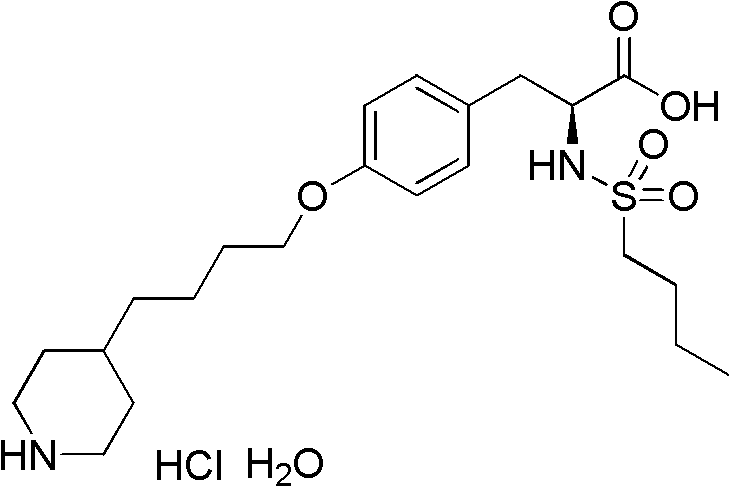
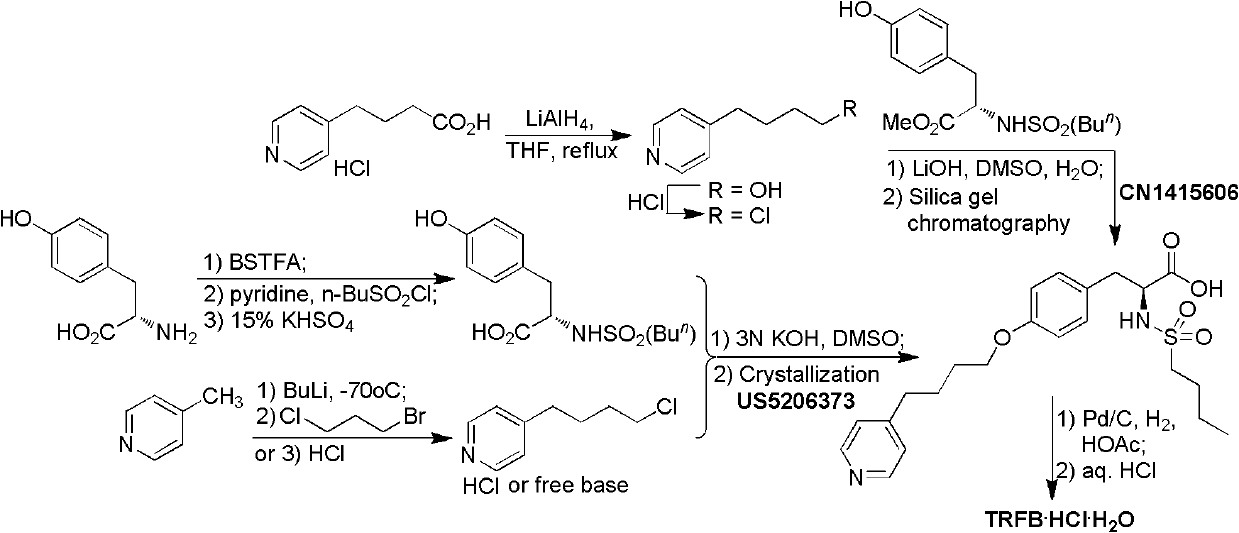
The preparation method of n-n-butylsulfonyl-o-(4-(4-pyridyl)-butyl)-l-tyrosinic acid alkyl ester
A technology of n-butylsulfonyl and tyrosine alkyl ester, which is applied in N-n-butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosine alkyl In the field of ester preparation, it can solve the problems of difficult scale-up production, long steps, and low total yield, and achieve the effects of avoiding environmental pollution, mild reaction conditions, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
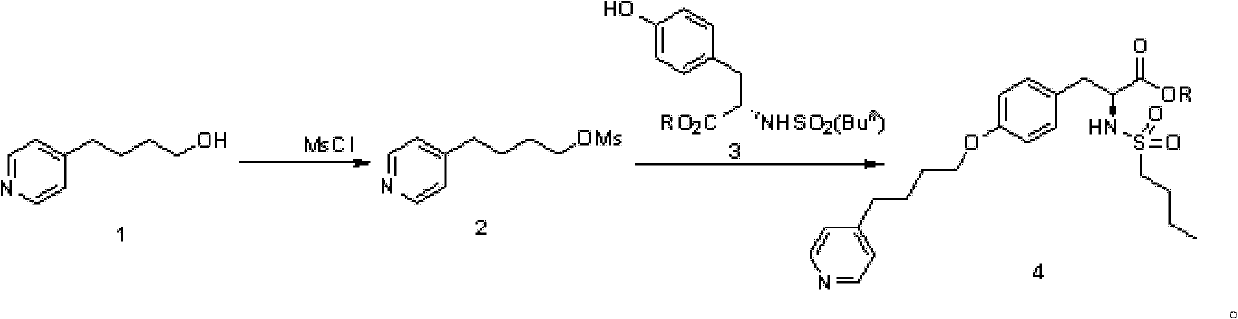
[0033] 1.1 Preparation of methylsulfonyl pyridine butanol (2)
[0034] A solution of 4-pyridinebutanol (1.5g, 10.0mmol) in dichloromethane (30mL) was cooled to 0°C, and triethylamine (2.1mL, 15mmol) and MsCl (3.5g, 30.6mmol) were added sequentially. Stir at 0°C for 10 minutes after the addition is complete. The reaction system was washed with anhydrous Na 2 SO 4 After drying and removing the solvent under reduced pressure, methanesulfonylpyridinebutanol (2) (2.46g, quant.) was obtained.
[0035] 1.2 Preparation of methylsulfonyl pyridine butanol (2)
[0036] A solution of 4-pyridinebutanol (8.0 g, 53.3 mmol) in dichloromethane (60 mL) was cooled to -5°C, and triethylamine (11.2 mL, 80.8 mmol) and MsCl (4.3 mL, 55.6 mmol) were added in sequence. Stir at 0°C for 10 minutes after the addition is complete. The reaction system was washed with anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain methanesulfonylpyridinebutanol (2) (12.50 g...
Embodiment 2
[0048] 2.1 N-Butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosine ethyl ester (4)
[0049] In N-n-butylsulfonyl-L-tyrosine ethyl ester (3.30g, 10.00mmol) solution in anhydrous DMF (30mL), the reaction system was sequentially added K 2 CO 3 (1.86g, 13.5mmol) and mesylpyridinebutanol (2) (2.46g, 10.73mmol). After the addition, the system was heated to 80°C and reacted for 12 hours. After routine post-treatment of the system, the target product N-n-butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosine ethyl ester 4 (1.5g, 32.5% ). 1 H NMR (300MHz, CDCl 3 ), δppm: 8.48(br, 2H), 7.13(d, J=4.8Hz, 2H), 7.10(d, J=9.0Hz, 2H), 6.80(d, J=8.7Hz, 2H), 5.10(d , J=9.0Hz, 1H), 4.33-4.18(m, 3H), 3.93(t, J=5.4Hz, 2H), 3.11-2.93(m, 2H), 2.79-2.66(m, 4H), 1.83- 1.81 (m, 4H), 1.66-1.56 (m, 2H), 1.34-1.23 (m, 5H), 0.86 (t, J=7.2Hz, 3H).
[0050] 2.2 N-butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosine ethyl ester (4)
[0051] In N-n-butylsulfonyl-L-tyrosine ethyl ester (16.33g, 49.57mmol) solution anh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com