A more stable sodium ozagrel compound and pharmaceutical composition thereof
A technology of ozagrel sodium and compounds, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of greater than 0.2%, and achieve the effects of good crystal shape, high yield, and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
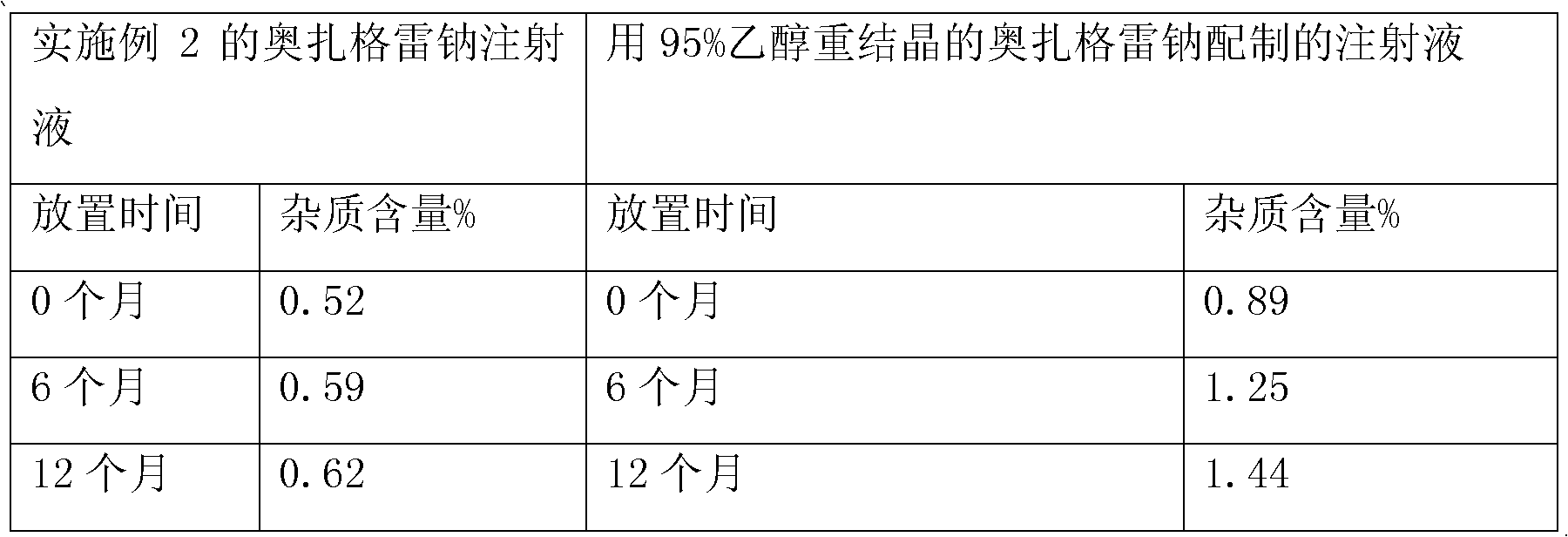
[0035] The crude product of sodium ozagrel is dissolved in a mixed solvent of ethyl acetate and methanol=1:1 (v / v) of 8 times its weight, and the active carbon of about 5% (w / v) weight of the solution volume is added, and heated to reflux Temperature, last for 1 hour, place at room temperature for 24 hours, precipitate crystals, obtain crystals after suction filtration, repeat the above steps to obtain the second recrystallization product, wash with ethanol, and dry to obtain pure ozagrel sodium, which is detected by HPLC , the obtained pure ozagrel sodium has a purity of 99.48%, and the content of two unknown impurities is less than 0.1%.
Embodiment 2
[0037]
[0038] Its preparation method is as follows:
[0039] Take 100 g of sodium ozagrel, mix and grind it with PEG-400, dissolve the ground material of sodium ozagrel and PEG-400 with the prepared disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH value of 6.5, and add 0.5% activated carbon, heated to 80°C and maintained for 15 minutes, filtered to remove carbon, then added sodium bisulfite, disodium edetate, stirred to dissolve, added disodium hydrogen phosphate-sodium dihydrogen phosphate buffer to 10000ml, added 0.5% activated carbon, boiled, finely filtered with a 0.45 μm microporous membrane, and sterilized with a 0.20 μm microporous membrane, canned, sterilized, and labeled.
Embodiment 3
[0041]
[0042] Its preparation method is as follows:
[0043] Take 100 g of sodium ozagrel, mix and grind it with PEG-400, dissolve the ground material of sodium ozagrel and PEG-400 with the prepared disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a pH value of 6.5, and add 0.5% activated carbon, heated to 80°C and maintained for 15 minutes, filtered to remove carbon, then added sodium bisulfite, disodium edetate, stirred to dissolve, added disodium hydrogen phosphate-sodium dihydrogen phosphate buffer to 10000ml, added 0.5% activated carbon, boiled, finely filtered with a 0.45 μm microporous membrane, and sterilized with a 0.20 μm microporous membrane, canned, semi-sealed, put into a freeze-drying box, freeze-dried, sealed, Label it and pack it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com