A kind of polyhydroxy demethyltropane compound and its preparation method and application
A compound, hydroxymethyl technology, applied in the field of polyhydroxy demethyltropane compounds and their preparation and application, can solve the problems of low yield and lengthy steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
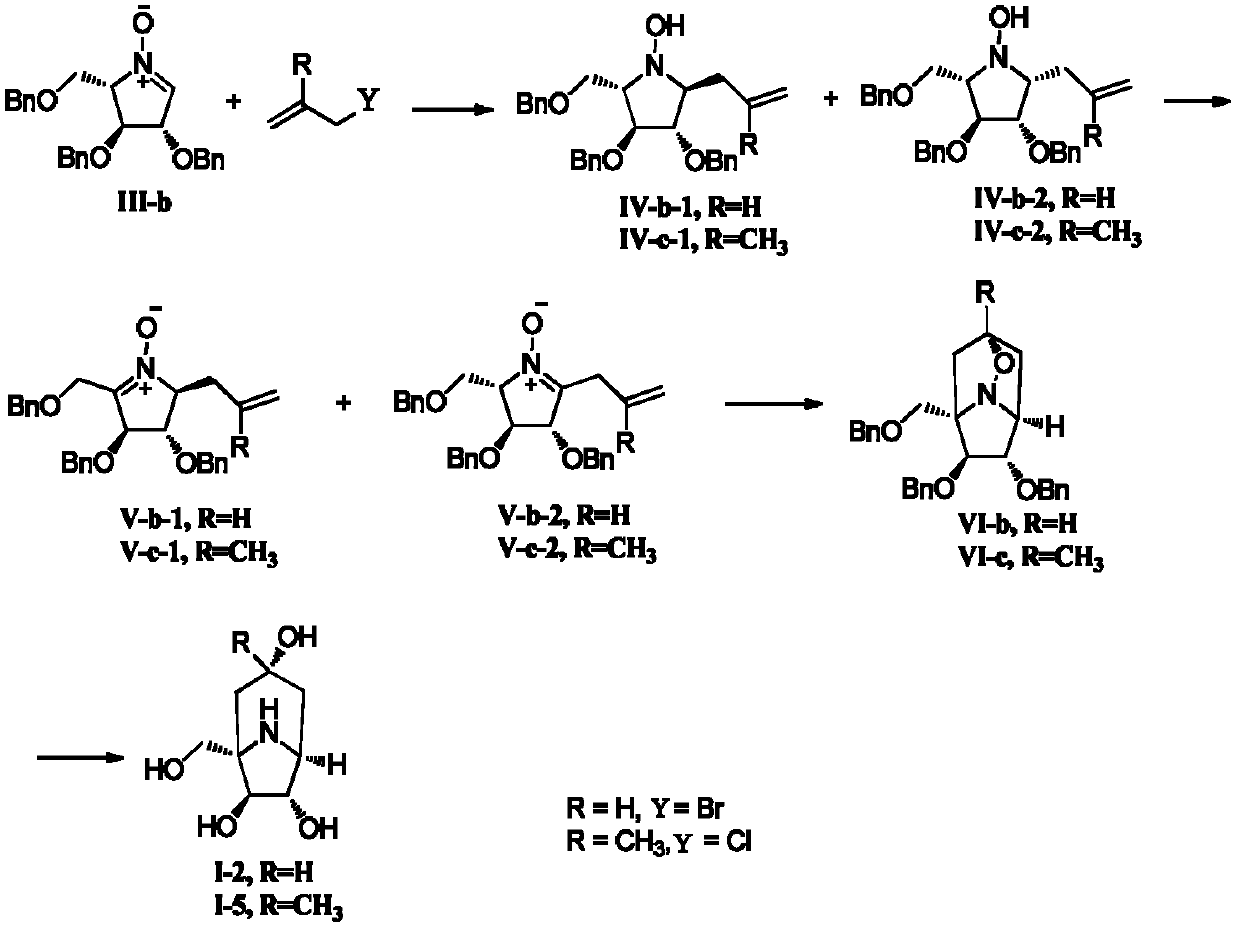
[0124] The preparation method of nitrone is illustrated with the preparation of III-b:
[0125] 1. Preparation of III-b
[0126] Add 10 ml of acetyl chloride dropwise to 500 ml of dry methanol under stirring in ice bath, then add D-xylose (30 g, 0.2 mol), keep the reaction at zero temperature until the raw materials disappear, neutralize with sodium bicarbonate to neutral , filtered to remove inorganic salts, and the crude product D-xylofuranoside obtained by evaporating the solvent to dryness was directly cast into the next step reaction.
[0127] Dissolve the crude product D-xylofuranoside (calculated according to 0.2mol) in dry DMF (200mL) from the previous step, and add NaH (29g, 0.72mol, content 60%) in THF (200mL) dropwise and DMF (200mL), after the addition, TBAI (2.0g) was added, and after half an hour, BnBr (0.66mol, 78.3mL) was added dropwise, and reacted until the raw material disappeared, then slowly added dropwise saturated ammonium chloride aqueous solution to q...
Embodiment 1
[0139] Embodiment 1: (1S, 3R, 5S, 6R, 7S)-1-(hydroxymethyl)-8-azabicyclo[3.2.1]octane-3,6 shown in synthetic formula (I-1) , 7-triol
[0140]
[0141] Under nitrogen protection conditions, allyl bromide (9.6mmol, 0.8mL) was slowly added dropwise to a solution of freshly ground magnesium chips (19.2mmol, 0.46g) in ether (9.6mL), and the solution was kept at In a slightly boiling state, a 1mol / L ether solution of allylmagnesium bromide was obtained. The above solution was slowly added to the THF solution of nitrone (Formula III-a) (1 g, 2.4 mmol) in an ice-water bath, and after half an hour of reaction, saturated ammonium chloride aqueous solution (5 mL) was added to quench the reaction, and at the same time, The organic phase was extracted with ethyl acetate (5 mL), the aqueous phase was extracted three times with ethyl acetate (10 mL each), and the combined organic phases were dried and concentrated. The resulting oil was subjected to silica gel chromatography (ethyl acet...
Embodiment 2
[0145] Embodiment 2: Compound (1S, 3R, 5S, 6S, 7S)-1-(hydroxymethyl)-8-azabicyclo[3.2.1]octane-3 shown in synthetic formula (I-2), 6,7-triol and the compound (1S, 3R, 5S, 6S, 7S)-1-(hydroxymethyl)-3-methyl-8-azabicyclo[3.2.1] shown in formula (I-5) ]octane-3,6,7-triol
[0146]
[0147]
[0148] Add allylmagnesium bromide ether solution (10mL) slowly to nitrone III-b (1.0g, 2.4mmol) tetrahydrofuran solution (15mL) under ice-water bath conditions, add saturated chloride solution after half an hour of reaction Aqueous ammonium solution (5 mL) was used to quench the reaction, and ethyl acetate (5 mL) was added to extract the organic phase. The aqueous phase was extracted three times with ethyl acetate (10 mL each), and the combined organic phases were dried and concentrated. The resulting oil was separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:12, v / v) to obtain (2S, 3S, 4S, 5S)-1-hydroxy-2-allyl-3,4- Bis(benzyloxy)-5-(benzyloxymethyl)pyrroli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com