Preparation method and product of piglet edema disease inactivated vaccine
A technology for piglet edema disease and inactivated vaccines, applied in the direction of microorganism-based methods, biochemical equipment and methods, medical preparations containing active ingredients, etc., can solve the problems of low number of cultured bacteria and high production costs, and achieve the goal of cultivating bacteria The effect of increasing the number, optimizing the production process and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
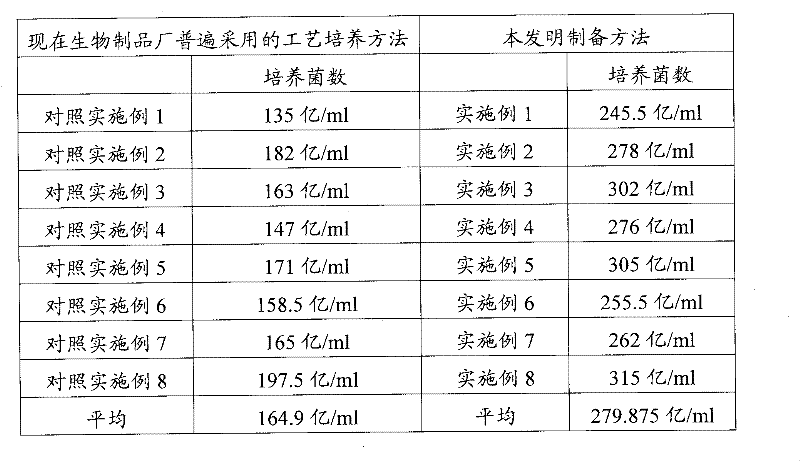
Image
Examples
Embodiment 1
[0028] 1. Preparation of primary slant strains: Escherichia coli C83684 strains were diluted and inoculated with synthetic plate medium for 22 hours at 37°C, and 10 typical colonies were selected to inoculate synthetic slant medium, cultivated at 37°C for 24h, to obtain primary slant strains, and placed for 4 ℃ refrigerator for use;
[0029] 2. Preparation of secondary strains: Inoculate the primary slant strains into the synthetic medium and incubate in a full-temperature shaker at 37°C for 16 hours. After the pure test confirms the sterility, obtain the secondary strains for use;
[0030] 3. Bacterial liquid culture: inoculate the secondary bacteria into the synthetic medium and cultivate it in a biological fermentation tank; the culture temperature is 37°C, the rotation speed is 100r / min, and the air intake volume is 18m 3 / h, the inner pressure of the tank is 0.05Mpa, and the incubation time is 12h; after the live bacteria are counted on the sampling plate, and the sterili...
Embodiment 2
[0033] 1. Preparation of primary slant strains: Escherichia coli C83684 strains were diluted and inoculated with synthetic plate medium for 24 hours at 37°C, and 20 typical colonies were inoculated into synthetic slant medium, cultivated at 37°C for 24 hours, to obtain primary slant strains, and placed for 4 ℃ refrigerator for use;
[0034] 2. Preparation of secondary strains: Inoculate the primary slant strains into the synthetic medium and incubate in a full-temperature shaker at 37°C for 16 hours. After the pure test confirms the sterility, obtain the secondary strains for use;
[0035] 3. Bacterial liquid culture: inoculate the secondary bacteria into the synthetic medium and cultivate it in a biological fermentation tank; the culture temperature is 37°C, the speed is 200r / min, and the air intake is 18m 3 / h, the inner pressure of the tank is 0.05Mpa, and the incubation time is 12h; after the live bacteria are counted on the sampling plate, and the sterility is confirmed b...
Embodiment 3
[0038] 1. Preparation of primary slant strains: Escherichia coli C83684 strains were diluted and inoculated with synthetic plate medium for 23 hours at 37°C, and 15 typical colonies were inoculated into synthetic slant medium, cultured at 37°C for 24h to obtain primary slant strains, and placed for 4 ℃ refrigerator for use;
[0039] 2. Preparation of secondary strains: Inoculate the primary slant strains into the synthetic medium and incubate in a full-temperature shaker at 37°C for 16 hours. After the pure test confirms the sterility, obtain the secondary strains for use;
[0040] 3. Bacterial liquid culture: inoculate the secondary strains into the synthetic medium and cultivate them in a biological fermentation tank; the culture temperature is 37°C, the rotation speed is 150r / min, the inner pressure of the tank is 0.05Mpa, and the culture time is 12h; during the fermentation process, Adopt the method of gradually increasing the ventilation volume, in which 0-2h static cultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com