Cefodizime sodium hydrate as well as preparation method and application thereof
A technology of cefodizime sodium and hydrate, which is applied in the field of medicine, can solve the problems such as the preparation method and application of cefodizime sodium crystalline hydrate that have not been reported in the literature, and achieve improved operability, good sliding properties, and prevention of adhesion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
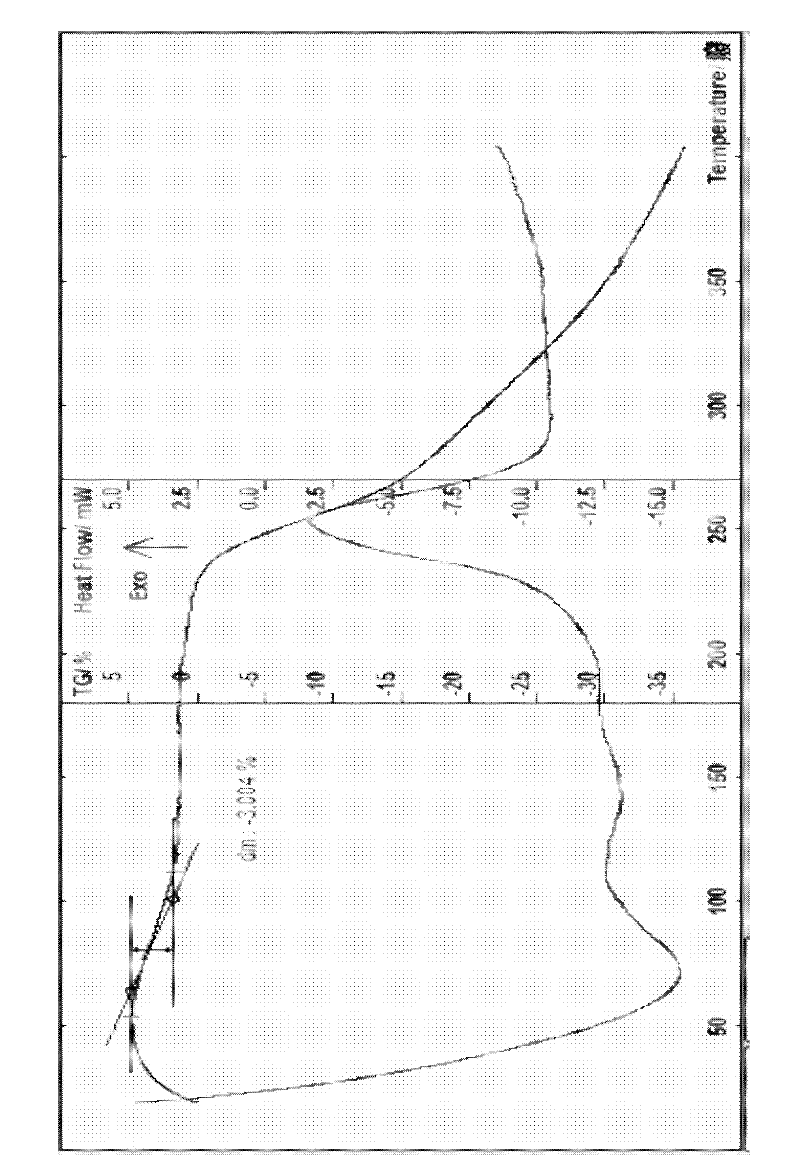
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of cefodizime sodium monohydrate Add 10 g of cefodizime acid and 30 ml of water into a reaction flask, stir to form a suspension, add 5 ml of triethylamine dropwise at 5°C, stir for 30 min, drop at 5°C Add 28% ethanol solution of sodium isooctanoate to make the pH to 6.9 (after adding too much, adjust the pH to about 6.9 with glacial acetic acid), stir, slowly add 50ml of acetone and 150ml of acetonitrile dropwise, and place it below -5°C to fully separate out the solid. Suction filtration, washing with a small amount of acetone for 3 times, suction filtration, the obtained solid was just dissolved with a small amount of water, added 0.15 g of activated carbon, stirred for 30 minutes, suction filtration, washing with water, suction filtration, and then recrystallized with 200 ml of acetonitrile and 200 ml of acetone, Place below 5°C to fully separate the crystals, filter with suction, wash with 30ml chloroform, filter with suction, and dry in vacuum...
Embodiment 2
[0067] Example 2 Preparation of cefodizime sodium monohydrate Add 10 g of cefodizime acid and 30 ml of water into the reaction flask, stir to form a suspension, add 3.8 ml of diethylamine dropwise at 5°C, stir to dissolve, add activated carbon 0.2g, stirred for 30 minutes, filtered with suction, washed with water, filtered with suction, added dropwise 28% ethanol solution of sodium isooctanoate to the filtrate at 5°C to make the pH to 6.9, stirred, slowly added to 400ml of acetone, placed below 0°C, Let the solid be fully separated, filter with suction, wash with a small amount of acetone for 3 times, filter with suction, dissolve the obtained solid with a small amount of water, recrystallize with 500ml of acetone, place it below -10°C, let the crystals fully separate out, filter with suction, 30ml Wash with chloroform, filter with suction, and dry under vacuum at 45°C for about 8 hours to obtain 5.6g of off-white crystals. 2.73%, which is within the error range with the sampl...
Embodiment 3
[0068] Example 3 Preparation of cefodizime sodium 1.5 hydrate Add 20 g of cefodizime acid and 50 ml of water into a reaction flask, stir to form a suspension, add dropwise a saturated aqueous solution of 3.8 g of anhydrous sodium carbonate at 5° C. to bring the pH to 7.0 (after adding more, adjust the pH to about 7.0 with glacial acetic acid), stir, add 0.3g of activated carbon, stir for 30 minutes, filter with suction, wash with water, filter with suction, slowly add 50ml of acetone and 250ml of isopropyl ether dropwise to the filtrate, 4°C Place below, let the solid fully separate out, filter with suction, wash with acetone 3 times, filter with suction, dissolve the obtained solid with a small amount of water, add 0.3g of activated carbon, stir for 30 minutes, filter with suction, wash with water, filter with isopropyl ether 100ml, 420ml of acetone to recrystallize it, place it below 5°C overnight, let the crystals fully separate out, filter with suction, wash with 30ml of ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com