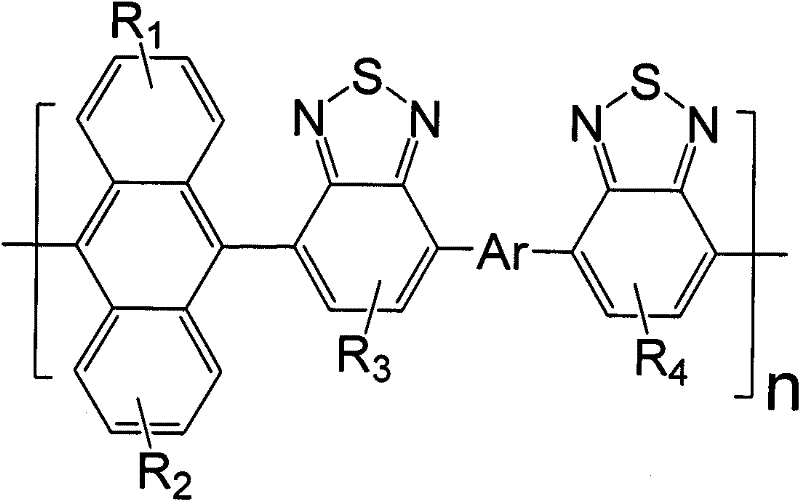
Anthracene and benzothiadiazole copolymer, and preparation method and application thereof
A technology of benzothiadiazoles and copolymers, which is applied in the field of organic compound synthesis, can solve the problems of mismatching device spectral response and solar radiation spectrum, ineffective use of red light region, and low collection efficiency of carrier electrodes. , to achieve high mobility, excellent reduction reversibility, and improved absorption coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
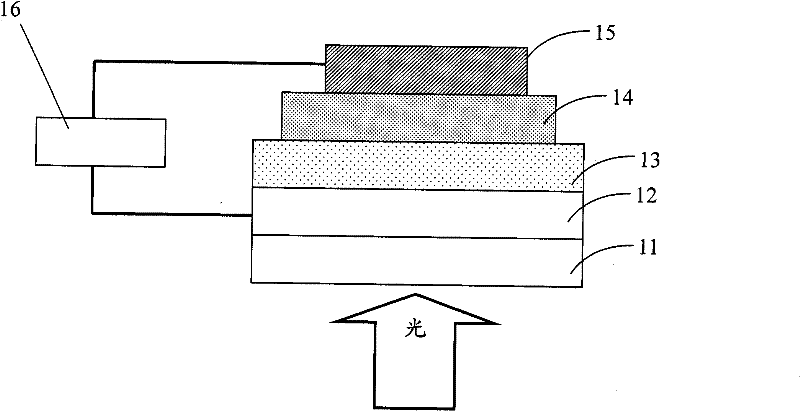
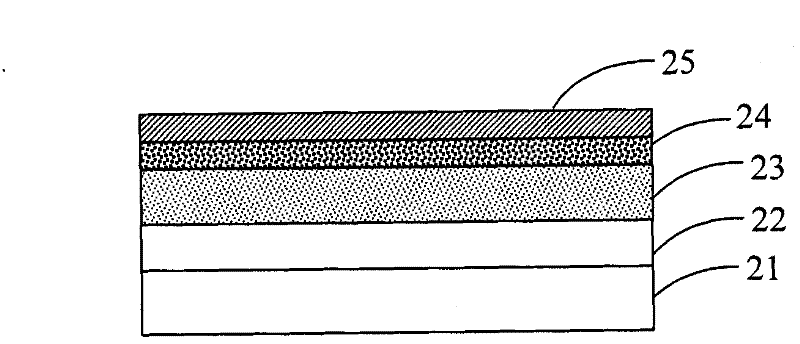
Image
Examples
preparation example Construction
[0040] And, the embodiment of the present invention also provides the preparation method of the copolymer containing anthracene and benzothiadiazole, including the following chemical reaction formula:
[0041]
[0042] That is, the specific process steps included are:
[0043] Compounds A and B represented by the following structural formula are provided respectively,
[0044] Among them, R 1 , R 2 selected from -H, cyano, halogen, or from substituted or unsubstituted C 1 ~C 40 Alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl; R 3 , R 4 selected from -H, cyano, or selected from substituted or unsubstituted C 1 ~C 40 Alkyl, substituted or unsubstituted C 1 ~C 40 Alkoxy, substituted or unsubstituted C 6 ~C 40 Aryl, substituted or unsubstituted C 6 ~C 40 Aralkyl, substituted or unsubstituted C 6 ~C 40 Arylalkoxy, Ar is an organic group with electron-donating properties;
[0045] In an oxygen-free and alkaline environment and i...
Embodiment 1
[0055] Containing the preparation of anthracene and benzothiadiazole copolymer PA-BFB, its structural formula is as follows:
[0056]
[0057] 1) The preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan) base-anthracene, its reaction is shown in the following formula:
[0058]
[0059] The specific process of preparation is: set up an anhydrous and anaerobic reaction device, and continuously stir and N 2 Under the protection of the three-necked flask, add 9.0mmol of light yellow crystal 9,10-dibromoanthracene, then inject 150ml of refined tetrahydrofuran solvent with a syringe, and then slowly inject 27.0mmol of n- BuLi reacted with each other, and the system gradually changed from light yellow to orange. After stirring the reaction for 2 hours, inject 30.6mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane with a syringe at -78°C, the system From orange to light yellow, warmed up to room temperature and reacted overnight. After the reaction, add satur...
Embodiment 2
[0067] Containing the preparation of anthracene and benzothiadiazole copolymer PODA-BFB, its structural formula is as follows:
[0068]
[0069] 1) 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan)-2,6-bis(2-octyldecyl)anthracene Preparation, its reaction is shown in the following formula:
[0070]
[0071] Set up an anhydrous and anaerobic reaction device, stirring and N 2 Under the protection of 9,10-dibromo-2,6-bis(2-octyldecyl)anthracene 5mmol into the three-necked flask, inject 150ml of refined tetrahydrofuran solvent with a syringe, and then re- Slowly inject 15mmol n-BuLi with a syringe, and stir for 2 hours; after 2 hours of reaction, inject 15mmol 2-isopropoxy-4,4,5,5-tetramethyl-1 with a syringe at -78°C , 3,2-dioxaborolane, warmed to room temperature and reacted overnight. After the reaction, add saturated aqueous sodium chloride solution, extract with chloroform, dry over anhydrous sodium sulfate, collect the filtrate after filtration and rotary evaporate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com