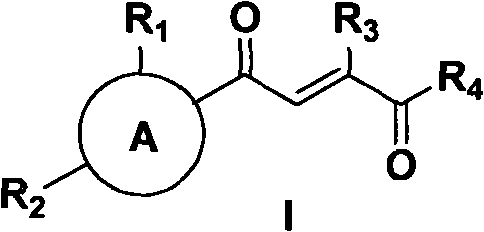
1-substituted-2-substituted-4- aryl substituted-butyl-2-alkene 1, 4-diketone compound, its preparation method and application
A compound, C1-C20 technology, applied in the field of medicine, can solve problems such as high acidity, difficulty in becoming a drug candidate compound, and poor ability to enter cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
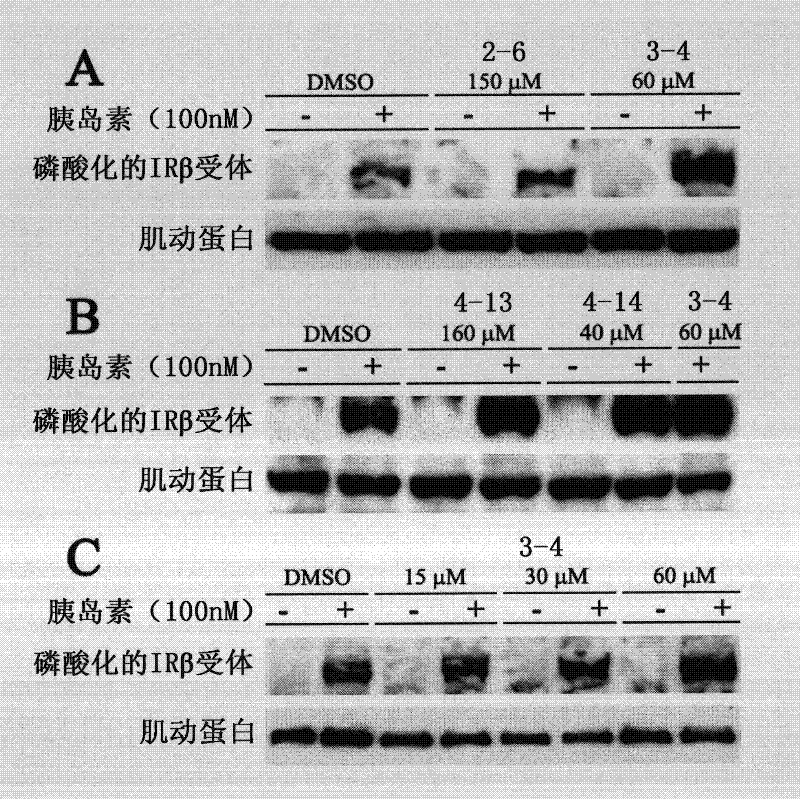
Image
Examples
Embodiment 1
[0111] Example 1: 4-[3-(2-Fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo-but-2-enoic acid (Compound 2-1)
[0112] Step 1: Preparation of 4-[3-(2-fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo-but-2-enoic acid methyl ester (Compound 1-1)
[0113]
[0114] Under the protection of nitrogen, add sodium methoxide (2.91g, 53.95mmol) and 45mL of anhydrous toluene to a dry reaction flask, cool to 0°C, add dimethyl oxalate (3.18g, 26.97mmol) and 1-[3- (2-Fluoro-benzyl)-phenyl]ethanone (2.461g, 10.79mmol) in ethylene glycol dimethyl ether solution 45mL was slowly added dropwise to the above system, stirred at 0℃ for 0.5 hours, and gradually heated to 60℃ After stirring overnight, the above system was quenched with 1N HCl solution, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, and anhydrous Na 2 SO 4 dry. It was concentrated to obtain 2.066 g of yellow oily compound 1-1, which was directly used in the next reaction.
[0115] Mp: 58-59°C. 1 ...
Embodiment 2
[0120] Example 2: Preparation of 4-[3-benzyloxyphenyl]-2-hydroxy-4-oxo-but-2-enoic acid methyl ester (Compound 1-2)
[0121]
[0122] Except that the compound 1-[3-benzyloxy)phenyl]ethanone was used in place of the 1-[3-(2-fluoro-benzyl)-phenyl]ethanone in step 1 of Example 1, the same as in Example 1 The same method as step 1 was used to prepare compound 1-2 (87%) as a pale yellow powder.
[0123] Mp: 70-71℃, 1 H NMR(400MHz, CDCl 3 ): δ15.22(br, 1H), 7.61-7.59(m, 2H), 7.47-7.34(m, 5H), 7.25-7.21(m, 2H), 7.08(s, 1H), 5.14(s, 2H) ), 3.96(s, 3H).EI-MS(M / Z): 312(M + ). Molecular formula C 18 H 16 O 5 Elemental analysis data: calculated value C 69.22, H 5.16, measured value C 69.18, H 5.19.
Embodiment 3
[0124] Example 3: Preparation of 4-[3-benzyloxyphenyl]-2-hydroxy-4-oxo-but-2-enoic acid (compound 2-2)
[0125]
[0126] Except that the compound 4-(3-benzyloxyphenyl)-2-hydroxy-4-oxo-but-2-enoic acid methyl ester was used instead of 4-[3-(2-fluoro-benzyl in step 2 of Example 1 Except for )-phenyl]-2-hydroxy-4-oxo-but-2-enoic acid methyl ester, the same method as in step 2 of Example 1 was used to prepare compound 2-2 (75.4%) as a pale yellow powder.
[0127] 1 H NMR(300MHz, CDCl 3 ): δ7.61-7.59 (m, 2H), 7.47-7.34 (m, 6H), 7.25-7.21 (m, 1H), 7.15 (s, 1H), 5.14 (s, 2H), EI-MS (M / Z): 298(M + ). Molecular formula C 11 H 14 O 5 Elemental analysis data: calculated value C 68.45, H 4.73, measured value C 68.52, H 4.38.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com