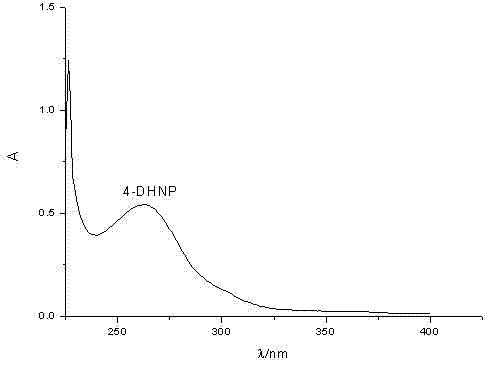
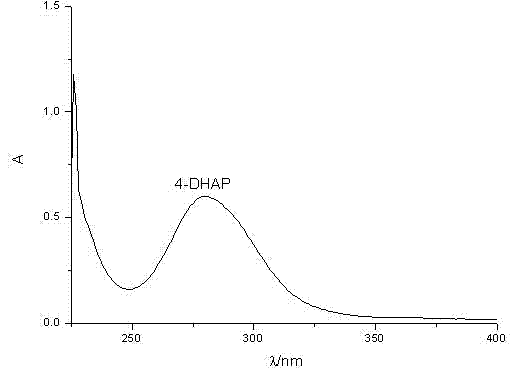
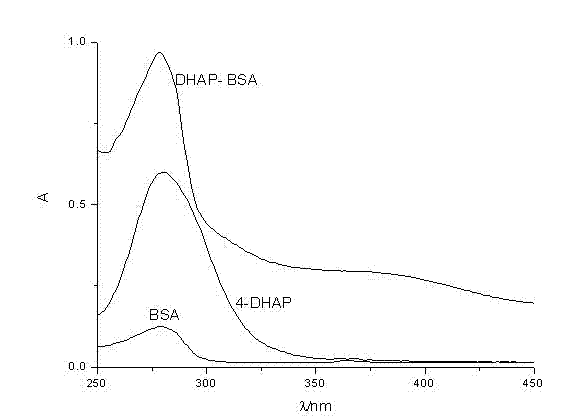
Synthetic method for 4-amino diheptyl phthalate
A technology of diheptyl aminophthalate and diheptyl nitrophthalate, which is applied in the field of synthesis of diheptyl 4-aminophthalate, can solve the problems of expensive instruments, cumbersome operations and limited method sensitivity and other problems, to achieve the effect of low cost, simple synthesis route and easy application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic method of embodiment 1 4-aminophthalate diheptyl ester
[0026] (1) Synthesis of diheptyl 4-nitrophthalate: Add 30ml of heptanol to 15g of 4-nitrophthalic acid, add 1.3ml of concentrated sulfuric acid as a catalyst under stirring conditions, and control the temperature at 120 o C, after stirring and refluxing for 12 hours, remove unreacted heptanol and water generated by reaction under reduced pressure. Pour it into ice water while it is hot, and add dropwise a 10% sodium carbonate solution with a mass fraction of 10% under stirring with a glass rod until the pH value of the water layer is neutral, then separate the oily product, extract the oily substance with absolute ethanol, and combine The extract was dried with anhydrous sodium sulfate, and then the ethanol was removed by rotary evaporation to obtain 9.75 g of diheptyl 4-nitrophthalate as a yellow oily liquid.
[0027] NMR identification of diheptyl 4-nitrophthalate:
[0028] 1 H NMR (300 MHz, CDC...
Embodiment 2
[0041] The synthetic method of embodiment 2 4-aminophthalate diheptyl ester
[0042] (1) Synthesis of diheptyl 4-nitrophthalate: Add n-heptanol to 4-nitrophthalic acid, the amount of n-heptanol and the amount of 4-nitrophthalic acid The ratio is 2:1. Add catalyst concentrated hydrochloric acid under stirring condition, the mass ratio of the amount of catalyst to 4-nitrophthalic acid is 1:7. Stir and reflux at 115°C for 10 hours. Evaporate the unreacted heptanol and the water generated by the reaction under reduced pressure, pour it into ice water while it is hot, and add a sodium carbonate solution with a mass fraction of 15% dropwise under stirring until the pH value of the water layer is 6.5, and the oily substance is separated After subsequent treatment, yellow oily liquid 4-nitrophthalate diheptyl ester was obtained;
[0043] (2) Synthesis of diheptyl 4-aminophthalate: add toluene to diheptyl 4-nitrophthalate, the mass ratio of toluene to diheptyl 4-nitrophthalate is 30...
Embodiment 3
[0044] The synthetic method of embodiment 3 4-aminophthalate diheptyl ester
[0045] (1) Synthesis of diheptyl 4-nitrophthalate: Add n-heptanol to 4-nitrophthalic acid, the amount of n-heptanol and the amount of 4-nitrophthalic acid The ratio is 3:1. Add catalyst concentrated sulfuric acid under stirring condition, the mass ratio of the amount of catalyst to 4-nitrophthalic acid is 2:7. Stir and reflux at 125°C for 13 hours. The unreacted heptanol and the water generated by the reaction were evaporated under reduced pressure, poured into ice water while it was hot, and a sodium carbonate solution with a mass fraction of 12 was added dropwise with stirring until the pH value of the water layer was 7.2, and the oil was separated by Subsequent processing to obtain yellow oily liquid 4-nitro-diheptyl phthalate;
[0046] (2) Synthesis of diheptyl 4-aminophthalate: Add benzene to diheptyl 4-nitrophthalate, and the mass ratio of benzene to diheptyl 4-nitrophthalate is 24 :1. Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com