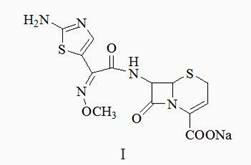
Preparation method of ceftizoxime sodium
A technology for ceftizoxime sodium and cephalosporin, which is applied in the field of drug synthesis, can solve problems such as harsh reaction conditions, many side reactions, and increase reaction steps, and achieves the effects of ensuring product quality and yield, simple operation, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
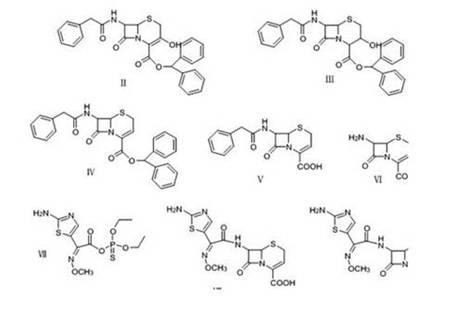
Image
Examples
Embodiment 1
[0050] Synthesis of 7-phenylacetamido-3-hydroxy-4-carboxylic acid-diphenylmethyl ester (Ⅲ)
[0051] Add 50.0g (0.1mol) of 7-phenylacetamido-3-hydroxy-3-cephalosporin-4-carboxylic acid-benzhydryl (II) into a 500ml double-necked bottle, and add 250ml of dichloromethane to dissolve. Cool down to -30°C, add sodium borohydride 4.54 (0.12mol), continue to cool down to -55~-50°C, and react for 1h. After adding 300ml of pure water and stirring, separate the liquid to take the organic phase, add 100ml of toluene, stir and crystallize, and filter with suction to obtain 46.0g of 7-phenylacetamido-3-hydroxy-4-carboxylic acid-diphenylmethyl ester (Ⅲ) product , yield 91.6%.
Embodiment 2
[0053] Synthesis of 7-phenylacetamido-3-non-3-cephalosporin-4-carboxylic acid (Ⅴ)
[0054] In a 1000ml reaction bottle, add 40.2g (0.08mol) of the reaction product from the previous step, and add 500ml of dichloromethane to dissolve. Cool down to -10°C, add 13.7g (0.12mol) methanesulfonyl chloride dropwise, stir for 15min, add 13.1g (0.13g) of triethylamine dropwise, keep warm at -10~-5°C for 30min, add dropwise 20.8ml of diethylamine (0.2mol), stirred at room temperature for 2.5h, added 300ml of pure water and stirred, separated the organic layer into a 1000ml reaction bottle, cooled to -10°C, added 60ml of sulfide anisole, and added trichloride dropwise under stirring A solution of aluminum (38g) in nitromethane (200ml) was stirred at room temperature for 5h, then 500ml of pure water was added and stirred for 15min, the liquid was separated, n-hexane was added to the organic phase, stirred and crystallized, and suction filtered to obtain the product 7-benzene Acetylamino-3-...
Embodiment 3
[0056] Synthesis of 7-amino-3-anol-3-cephalosporin-4-carboxylic acid (Ⅵ)
[0057] In a 250ml reaction flask, add 12.7g (0.04mol) of 7-phenylacetamido-3-ceph-4-carboxylic acid-diphenylmethyl ester, add 80ml of dichloromethane, cool to -15°C, and add dimethyl Amine 4.0ml (0.06mol), dimethyl disulfide 15ml, stirring and dissolving, add dropwise 60ml of phosphorus pentachloride 12.5g (0.06mol) in dichloromethane solution, stir and react at -5°C for 1h, cool to -20°C ℃, 50ml of isobutanol was added dropwise, and reacted at room temperature for 3h. Extract twice with 300ml of pure water, combine the water phases, decolorize with 1g of activated carbon, adjust the pH to 4 with ammonia water, stir and crystallize, and filter with suction to obtain 7.4g of the product 7-amino-3-non-3-cephalosporin-4-carboxylic acid , yield 92.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com