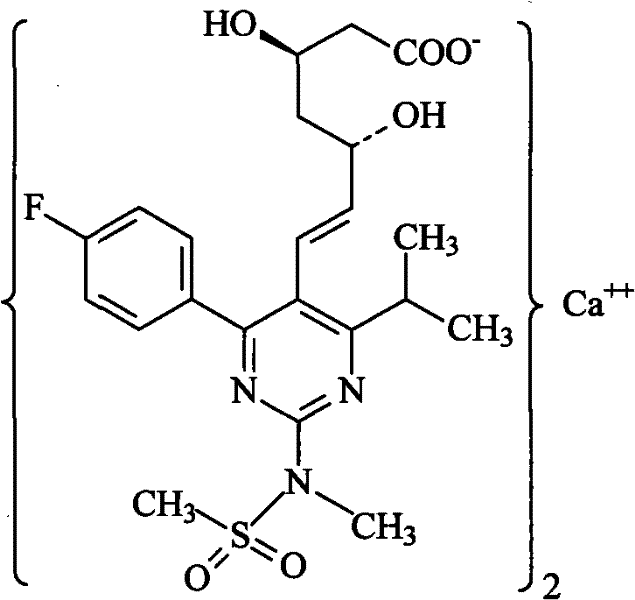
Method for preparing rosuvastatin calcium
A technology for rosuvastatin and compounds, which is applied in the field of pharmaceutical preparation, can solve the problems of expensive reagents, harsh reaction conditions and the like, and achieves the effects of stable yield, easy operation and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
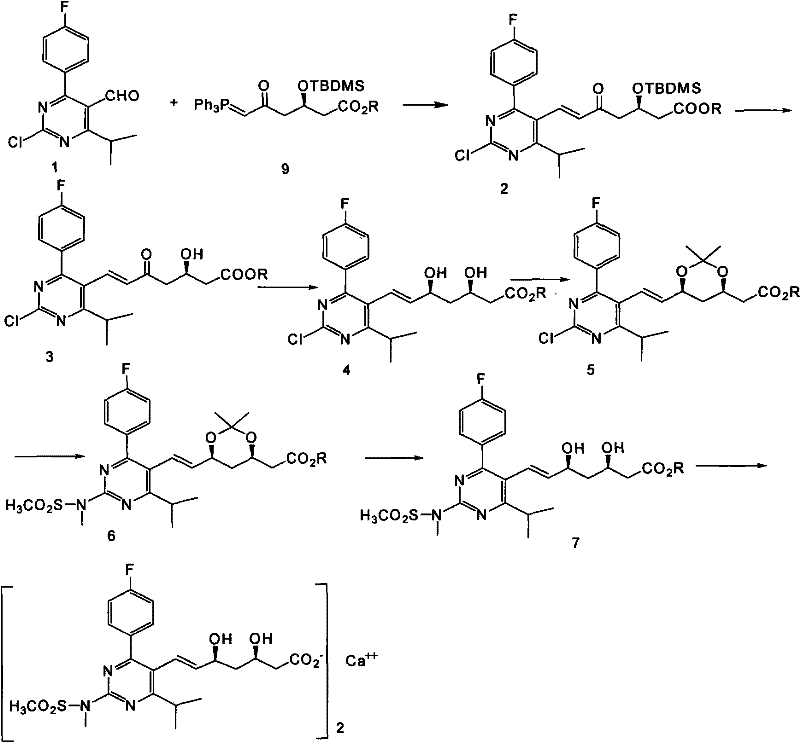
[0024] (R,E)-3-(tert-butyl-dimethyl-silyloxy)-7-(2-chloro-4-(4-fluorophenyl)-6-isopropyl-pyrimidine-5 Preparation of -yl)-5-oxo-6-heptenoic acid methyl ester (compound 2).
[0025] Reaction formula:
[0026]
[0027] Steps:
[0028] Add 20 grams of compound 1, 58 grams of phosphorus ylide reagent and 500 mL of anhydrous acetonitrile to a 1L single-necked bottle, and heat to reflux for 20 hours. The reaction is complete as monitored by TLC. The solvent is removed by rotary evaporation in a water bath at 30 ° C. The residue is washed twice with 20 mL of petroleum After washing with ether, 28.8 g of the compound was obtained, which was directly used in the next reaction with a yield of 74.95%.
[0029] 1 HNMR (300MHz, CDCl 3 ): δ0.01 (6H, d), 0.78 (6H, s), 1.26 (6H, dd, J = 6.69Hz), 2.47 (2H, m), 2.74 (2H, dd, J = 6.02Hz), 3.3 (1H, m), 3.65 (3H, s), 4.58 (1H, m), 6.19 (1H, dd, J=16.42Hz), 7.11 (2H, m), 7.56 (3H, m).
[0030] MS (ESI) m / z: 535 (M+H), 557 (M+Na).
Embodiment 2
[0032] (R,E)-7-(2-Chloro-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl)-3-hydroxy-5-oxo-6-heptenoic acid Preparation of methyl ester (compound 3).
[0033] Reaction formula:
[0034]
[0035] Steps:
[0036] In a 500mL single-necked bottle, add 18g of the compound of formula 2, 30mL of tetrahydrofuran, 90mL of glacial acetic acid and 30mL of water, and heat to 40°C for 24 hours. TLC monitors that the reaction is complete. Extract three times with 150ml of ethyl acetate, combine the oil layers, and wash with water. Monitored by TLC, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated in a water bath at 30°C to remove the solvent to obtain 13.45 g of compound 3, which can be directly used in the next reaction with a yield of 95%.
[0037] 1 HNMR (300MHz, CDCl 3 ): δ1.29 (6H, dd, J = 6.7Hz), 2.52 (2H, dd, J = 6.32Hz), 2.72 (2H, m), 3.34 (1H, m), 3.7 (3H, s), 4.46 (1H, m), 6.20 (1H, dd, J=16.42Hz), 7.09-7.15 (2H, m), 7.53-7.60 (3H, m)...
Embodiment 3
[0039] (3R,5S,E)-7-(2-Chloro-4-(4-fluorophenyl)-6-isopropyl-pyrimidin-5-yl)-3,5-dihydroxy-6-heptenoic acid Preparation of methyl ester (compound 4).
[0040] Reaction formula:
[0041]
[0042] Steps:
[0043] In a 2000mL three-neck flask, under argon protection, add 24.2g of the compound of formula 3, 650mL of anhydrous tetrahydrofuran, 170mL of anhydrous methanol, cool to -50°C, add 12.2g of 50% diethylmethoxyboron reagent, and react for 0.5 hour, add 2.67 g of sodium borohydride, control the temperature at -40°C--60°C for 3 hours, TLC monitors that the reaction is complete, add 33mL of glacial acetic acid to quench the reaction, adjust the pH value to about 8 with saturated aqueous sodium carbonate solution, 240 ml Ethyl acetate was extracted three times, the oil layers were combined, washed with water, monitored by TLC, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation in a water bath at 30°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com