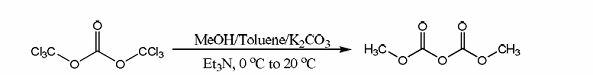Preparation method for dimethyl dicarbonate
A technology of dimethyl dicarbonate and methyl chloroformate, which is applied in the field of preparation of dimethyl dicarbonate, can solve the problems of not being suitable for actual production, low product purity, long reaction time, etc., and it is not easy to achieve The effect of complete removal, simple process, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: In a 2000 ml reaction flask, add 400 g of methyl chloroformate (technical grade), 400 ml of dichloromethane, add 143.82 g of dodecyl dimethyl benzyl ammonium chloride, stir, and cool to 5~ 15°C, add 1166g of 14% sodium hydroxide aqueous solution dropwise, after the dropwise addition, let stand to separate layers, discard the water layer, add 32g of 85% sulfuric acid to the organic layer, stir at room temperature for 1.5 hours, separate layers, add anhydrous magnesium sulfate to the organic layer to dry , filtered, then distilled under reduced pressure, first remove the solvent, and then collect the fraction at 200Pa, 30°C-35°C to obtain 232g of colorless liquid product dimethyl dicarbonate, yield 81.9%, freezing point 17°C, GC purity 99.9 %.
[0028] The acid and alkali solution concentrations mentioned in this embodiment are all mass percentage concentrations, the same below.
[0029] In this example, tri-(dodecyl)methyl ammonium chloride, tri-(tetradecyl)m...
Embodiment 2
[0030] Embodiment 2: in 2000 milliliter reaction bottle, add 400g methyl chloroformate (industrial grade), 380 milliliters of ethylene dichloride, add 11.14g tri-(dodecyl) methyl ammonium chloride, stir, cool to 0~10°C, add 1420g15.8% potassium hydroxide aqueous solution dropwise, after the dropwise addition is completed, let it stand for stratification, add 100g30% hydrochloric acid to the organic layer, stir at room temperature for 1 hour, and separate layers, add anhydrous magnesium sulfate to the organic layer, Dry for 1 hour, filter off the desiccant, distill off the solvent under reduced pressure, and then collect the fraction at 200 Pa, 30°C-35°C to obtain 241 g of colorless liquid product dimethyl dicarbonate, with a yield of 85.2% and a freezing point of 17°C. 99.9% pure.
Embodiment 3
[0031] Example 3: In a 2000ml reaction flask, add 400g methyl chloroformate (technical grade), 400ml benzene, add 10g tri-(tetradecyl)methylammonium chloride, stir, and cool to 15-25°C , add dropwise 1600g of 12.8% potassium hydroxide aqueous solution, dropwise addition, layering, add 30g90% sulfuric acid to the organic layer, stir at room temperature for 1 hour, layering, add anhydrous magnesium sulfate to the organic layer, dry at room temperature for 1 hour, filter off Desiccant, distilled under reduced pressure to remove the solvent, and then collected the fraction at 200Pa, 30°C-35°C to obtain 232g of a colorless liquid product, dimethyl dicarbonate, with a yield of 82.1%, a freezing point of 17°C, and a purity of 99.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com