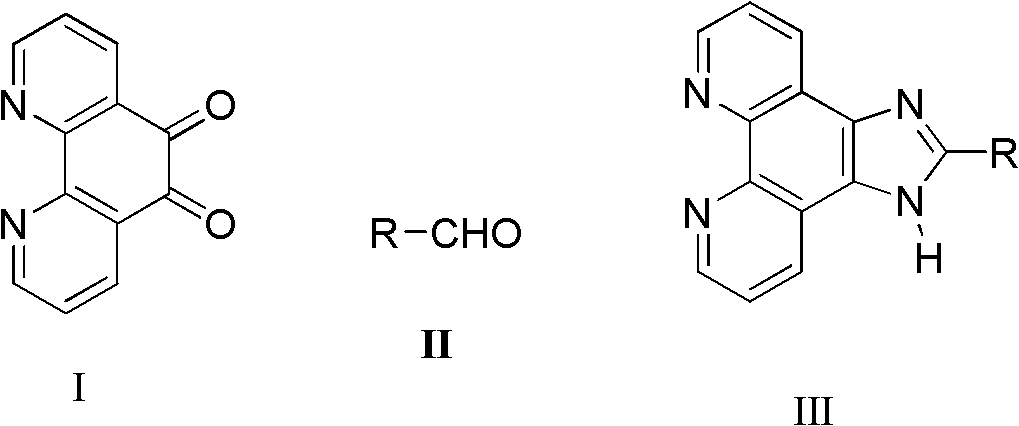
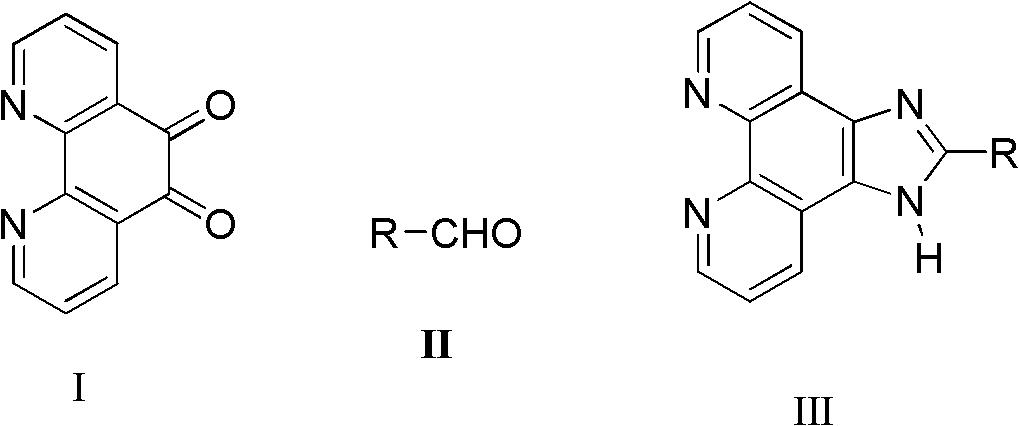
Synthetic process of imidazo phenanthroline compound
A synthesis process and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of a large number of waste acids and complicated operations, and achieve the effects of avoiding the use of acids, high separation efficiency, and energy-saving production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1,10-phenanthroline-5,6-dione (0.105g, 0.5mmol), ammonium acetate (0.154g, 2mmol) and benzaldehyde (0.0648g, 0.6mmol) successively in a mortar, Grind, make it fully mixed, react at a constant temperature at 85°C for 9 hours, after the reaction is detected by TLC, cool to room temperature, wash with distilled water, adjust pH=7 with concentrated ammonia water, filter with suction, dry, and recrystallize with absolute ethanol to obtain Light yellow powdery solid product (0.1275g, 89.8%): m.p.>300°C (literature value>300°C); 1 H NMR (500MHz, DMSO-d 6 ), δ (ppm): 13.79 (s, 1H, NH), 9.06 (d, J = 1.0Hz, 2H), 8.94 (dd, J1 = 1.0Hz, J 2 =8.0Hz, 2H), 8.33-8.29(m, 2H), 7.90-7.53(m, 5H, ph-H).
Embodiment 2
[0025] Add 1,10-phenanthroline-5,6-dione (0.105g, 0.5mmol), ammonium acetate (0.077g, 1mmol) and benzaldehyde (0.0648g, 0.6mmol) successively in a mortar, Grind, make it fully mixed, react at a constant temperature at 85°C for 9 hours, after the reaction is detected by TLC, cool to room temperature, wash with distilled water, adjust pH=7 with concentrated ammonia water, filter with suction, dry, and recrystallize with absolute ethanol to obtain The product was a pale yellow powdery solid (0.1275 g, 80.5%).
Embodiment 3
[0027] Add 1,10-phenanthroline-5,6-dione (0.105g, 0.5mmol), ammonium acetate (0.154g, 2mmol) and benzaldehyde (0.0648g, 0.6mmol) successively in a mortar, Grind it, make it fully mixed, react at a constant temperature at 60°C for 8 hours, after the reaction is detected by TLC, cool to room temperature, wash with distilled water, adjust pH=7 with concentrated ammonia water, filter with suction, dry, and recrystallize with absolute ethanol to obtain The product was a pale yellow powdery solid (0.1178 g, 74.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com