Method for preparing electrocatalytic noble metal nanomaterial with three-dimensional network structure
An electrocatalytic material and network structure technology, applied in the field of preparation of noble metal nano-electrocatalytic materials, can solve the problems of not being widely applicable to a variety of noble metals and cumbersome preparation methods, and achieve adjustable size, simple equipment, and improved hydrogen storage performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh 0.005 gram of palladium acetate and 0.01 gram of DTAB (dodecyltrimethylammonium bromide) and put it into a reaction kettle with a volume of 50 ml (the mass ratio of palladium acetate and DTAB is 1: 2), add 20 ml of ethylene glycol (to make the mass fraction of palladium acetate 0.0224%), and magnetically stir to form a homogeneous suspension.
[0026] (2) Add 0.05 ml of formaldehyde to the stirred suspension, and magnetically stir for 5 minutes.
[0027] (3) The reaction kettle was sealed, placed in an oven, and reacted at 150° C. for 8 hours.
[0028] (4) Take out the reactor and cool it down to room temperature naturally.
[0029] (5) Transfer the reacted product from the reaction kettle to a centrifuge tube, alternately centrifuge and ultrasonically wash the product with acetone and absolute ethanol, repeat 5 times, and obtain pure three-dimensional network structure palladium.
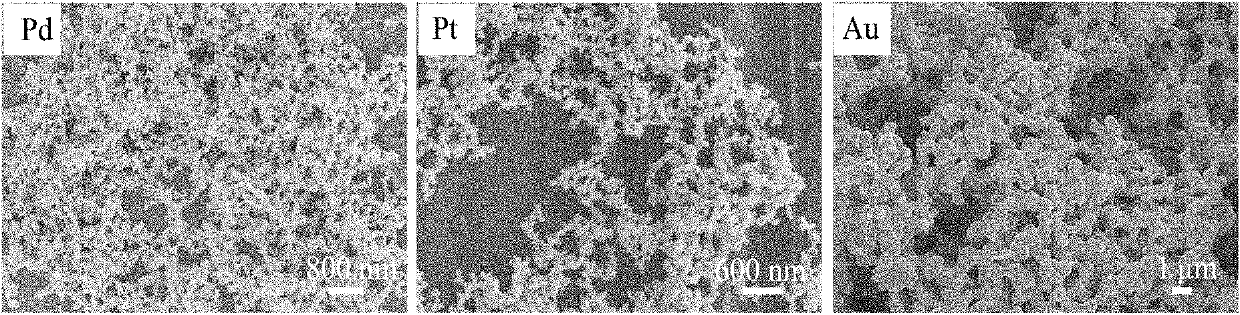
[0030] The SEM photograph of embodiment 1 is attached figure 1 The first ph...
Embodiment 2
[0032] (1) Weigh 0.01 gram of palladium acetate and 0.02 gram of DTAB and put it into a 25 ml reaction kettle (the mass ratio of palladium acetate and DTAB is 1:2), add 10 milliliters of ethylene glycol (make the mass fraction of palladium acetate 0.0896%), magnetically stirred to form a homogeneous suspension.
[0033] (2) Add 0.1 ml of formaldehyde to the stirred suspension, and magnetically stir for 5 minutes.
[0034] (3) The reaction kettle was sealed, placed in an oven, and reacted at 150° C. for 8 hours.
[0035] (4) Take out the reactor and cool it down to room temperature naturally.
[0036] (5) Transfer the reacted product from the reaction kettle to a centrifuge tube, alternately centrifuge and ultrasonically wash the product with acetone and absolute ethanol, repeat 5 times, and obtain pure three-dimensional network structure palladium.
[0037] The SEM photo of Example 2 is similar to that of Example 1, but the size of the basic particle unit constituting the ne...
Embodiment 3
[0039] (1) Take by weighing 0.02 gram of palladium acetate and 0.04 gram of DTAB and put into a volume of 25 milliliters of reactor (the mass ratio of palladium acetate and DTAB is 1: 2), add 5 milliliters of ethylene glycol (the massfraction of palladium acetate is 0.3584%), magnetically stirred to form a homogeneous suspension.
[0040] (2) Add 0.2 ml of formaldehyde to the stirred suspension, and magnetically stir for 5 minutes.
[0041] (3) The reaction kettle was sealed, placed in an oven, and reacted at 150° C. for 8 hours.
[0042] (4) Take out the reactor and cool it down to room temperature naturally.
[0043] (5) Transfer the reacted product from the reaction kettle to a centrifuge tube, alternately centrifuge and ultrasonically wash the product with acetone and absolute ethanol, repeat 6 times, and obtain pure three-dimensional network structure palladium.
[0044] The SEM photo of Example 3 is similar to that of Example 1, but the size of the basic particle unit co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com