Tapasin augmentation for enhanced immune response
An immune response and enhancer technology, applied in vaccines, medical preparations containing active ingredients, and the use of vectors to introduce foreign genetic material, etc., can solve the problems of reducing the expression of MHC molecules and the instability of MHC class I carrier complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The invention is now further illustrated in the following detailed examples, which are presented as illustrative only and should not be construed as otherwise limiting the scope or spirit of the invention or any embodiment thereof.
[0015] Materials and methods
[0016] Cells, Viruses, and Mice
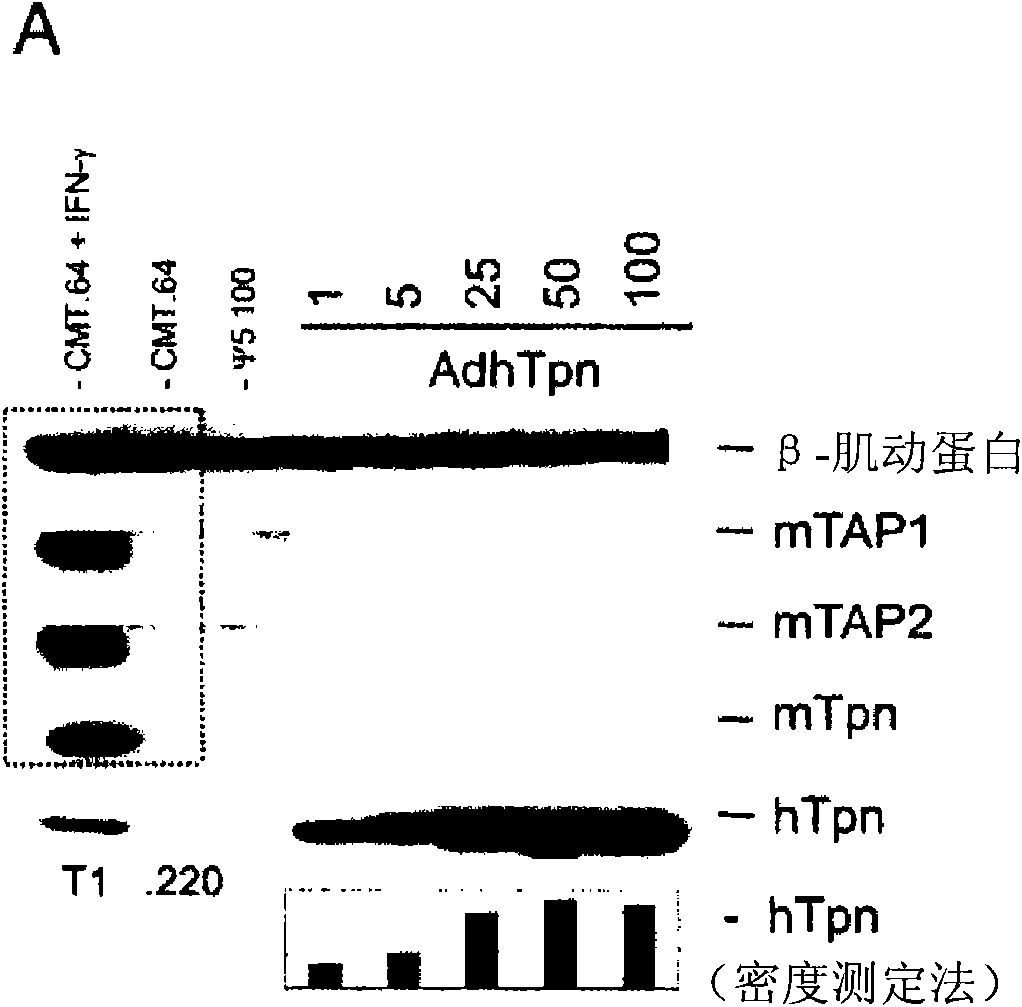
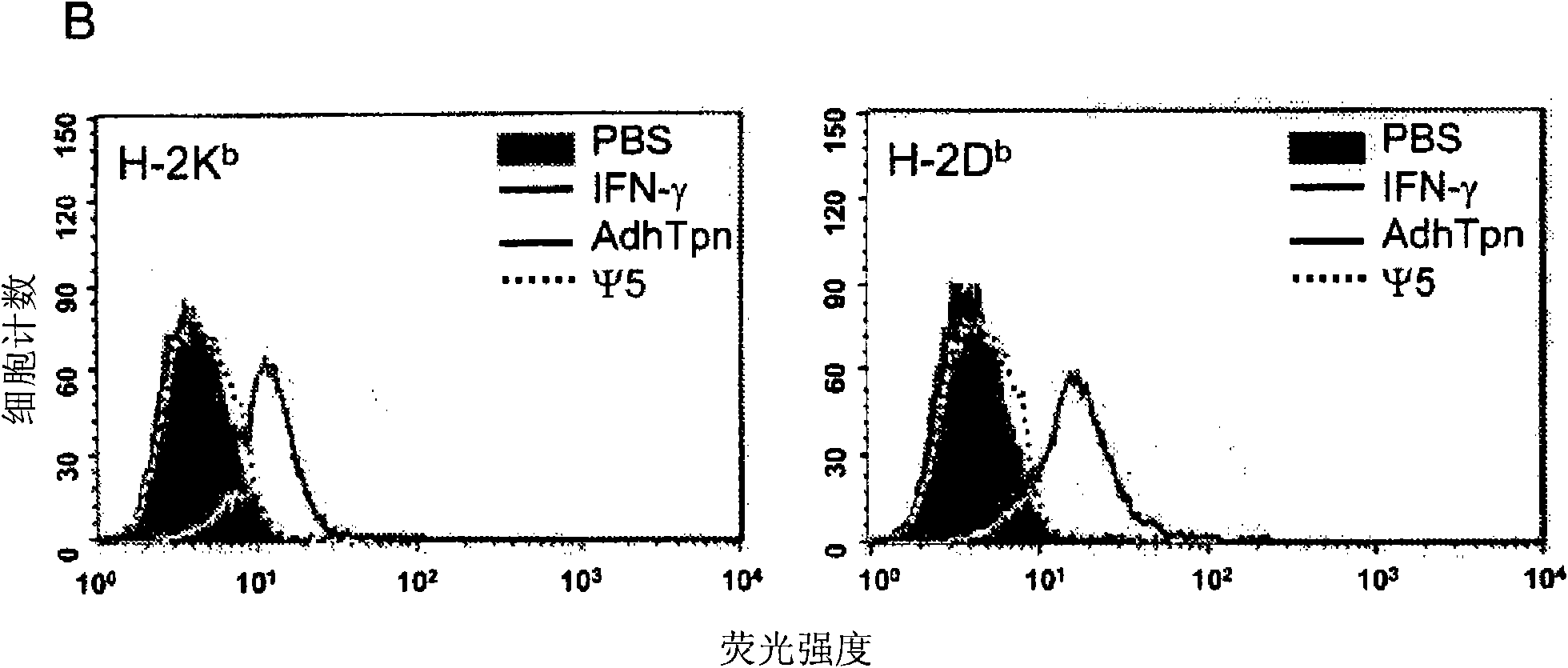
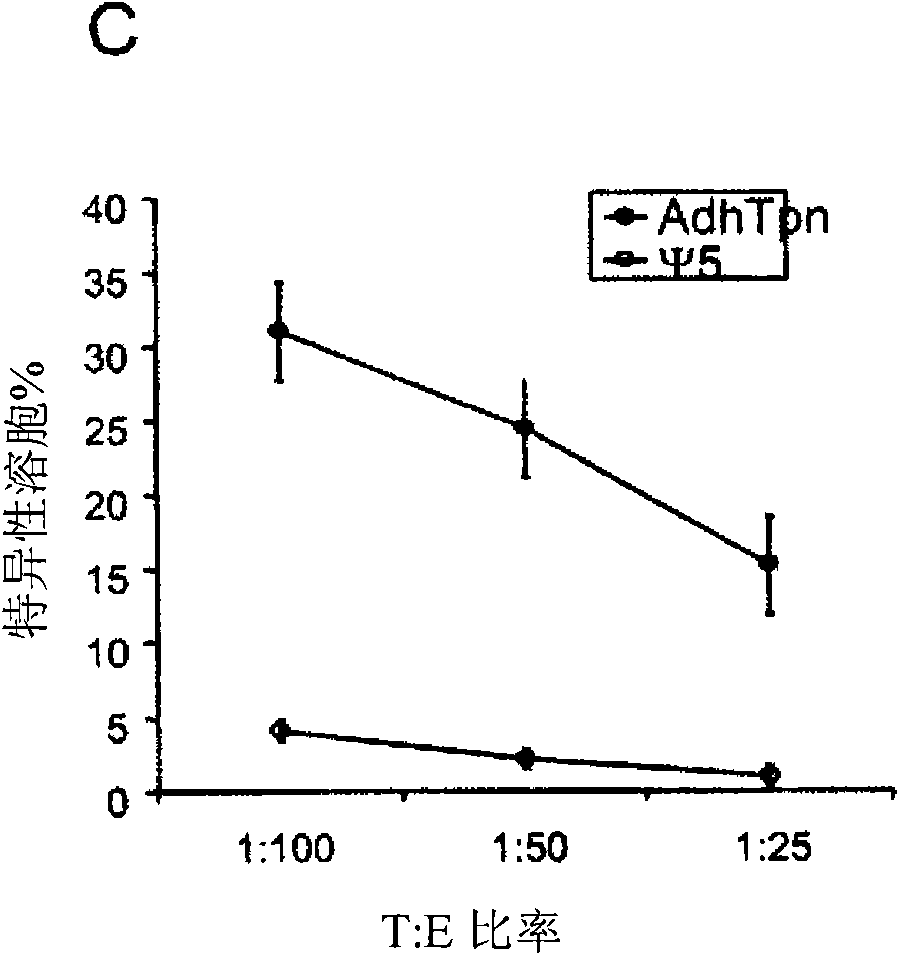
[0017] HEK 293 cells (ATCC, Rockaway, MD, USA), CRE8 cells (S. Hardy (S. J.Virol; 71:1842-1849 (1997)), CMT.64 cells (Y.Lou et al.; Cancer Res.; 65 : 7926-7933 (2005)), CMT / VSV-NP (CMT.64 cells transfected with the VSV nucleocapsid protein (NP) minigene containing b Immunodominant epitope of amino acids 52 to 59 on ) and T1 (ATCC, CRL-1991, hTpn positive cell line). CRE8 cells have a β-actin-based expression cassette that utilizes an N-terminal nuclear localization signal to drive stable integration of the Cre recombinase gene into HEK 293 cells (S. Hardy et al., supra). The Ψ5 virus is an E1 and E3 deleted form of Ad5 containing loxP sites flanking the assembly site (S. H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com