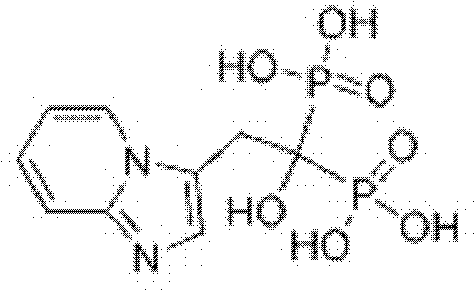
Minodronate tablets and preparation method thereof
A technology of minodronic acid tablets and minodronic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large production fault tolerance and achieve rapid absorption Complete, rapid onset of action, enhanced dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 (prescription technology that does not use solid dispersion technology)
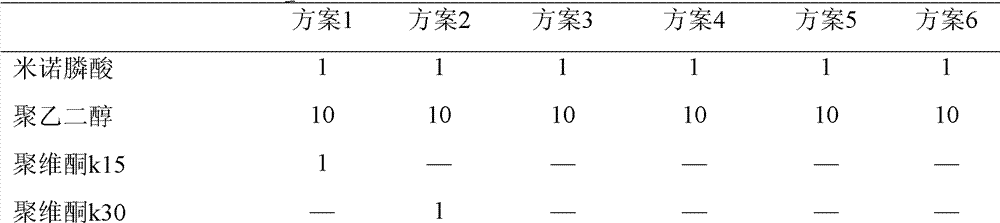
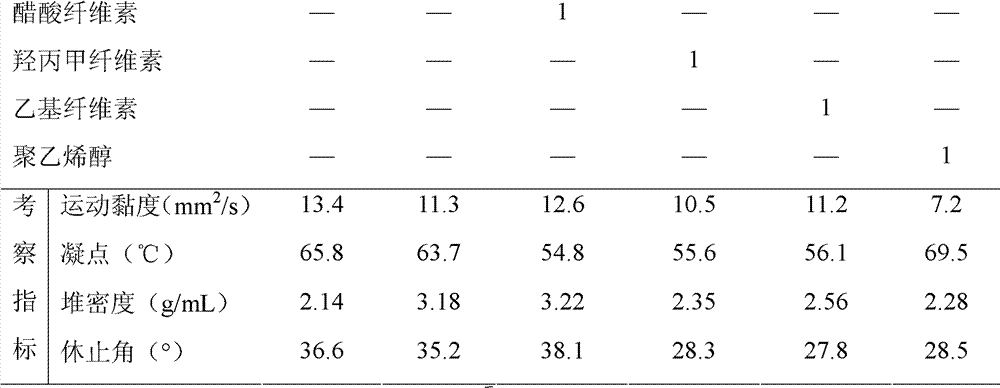
[0046] Prescription (1000 tablets):
[0047]
[0048] Preparation Process:
[0049] 1), take the minodronic acid, lactose, microcrystalline cellulose, croscarmellose sodium and magnesium stearate of prescription quantity, sieve, mix homogeneously;
[0050] 2), compress into tablets to obtain minodronic acid tablets.
Embodiment 2
[0051] Embodiment 2 (prescription technology that does not add additive)
[0052] Prescription (1000 tablets):
[0053]
[0054] Preparation Process:
[0055] 1) Preparation of solid dispersion: heat the prescribed amount of polyethylene glycol to 80°C and keep it warm until completely melted, then add the prescribed amount of minodronic acid, stir until completely dissolved, continue stirring and cool to 0~- Place at 4°C, 0~-4°C for 24 hours to make it brittle, crush it, and pass through a 60-mesh sieve to obtain a solid dispersion of minodronic acid;
[0056] 2), take microcrystalline cellulose, lactose, crospovidone and magnesium stearate of recipe quantity, mix with minodronic acid solid dispersion;
[0057] 3), compressing into tablets to obtain minodronic acid tablets.
Embodiment 3
[0059] Prescription (1000 tablets):
[0060]
[0061] Preparation Process:
[0062] 1) Preparation of solid dispersion: heat the prescribed amount of polyethylene glycol to 80°C and keep it warm until completely melted, slowly add polyvinyl alcohol, stir until dissolved, then add the prescribed amount of minodronic acid, stir until completely dissolved Afterwards, continue to stir and cool to 0~-4°C, place it under the condition of 0~-4°C for 24 hours to make it solid and brittle, crush it, pass through a 60-mesh sieve, and obtain the minodronic acid solid dispersion;
[0063] 2), take microcrystalline cellulose, lactose, croscarmellose sodium and magnesium stearate of recipe quantity, mix with minodronic acid solid dispersion;
[0064] 3), compressing into tablets to obtain minodronic acid tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com