Preparation method of microcapsule vaccines capable of resisting infection of aeromonas hydrophila
A vaccine preparation technology for Aeromonas hydrophila, which is applied in the field of vaccine preparation for the prevention and treatment of fish bacterial sepsis, and can solve the problems of time-consuming and labor-intensive, unfavorable large-scale promotion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of Aeromonas hydrophila suspension
[0029] Under sterile conditions, inoculate the pathogenic Aeromonas hydrophila strain S1 of Acipenser siberian bacterial septicemia stored on the slant medium of the test tube into the common nutrient broth with pH 7.2, shake at 30°C and 150 rpm Centrifuge at 3000 rpm for 20 min after shaking the bed for 48 h, wash with sterile saline for 3 times, and make the effective viable bacteria content of 10 10 siberian sturgeon bacterial septicemia pathogenic Aeromonas hydrophila strain S1 bacterial suspension, inactivated with formalin, and stored in a refrigerator at 4°C.
Embodiment 2
[0031] Preparation of microcapsule vaccine against Aeromonas hydrophila
[0032] Mix 200 ml of the inactivated Siberian sturgeon bacterial septicemia pathogenic Aeromonas hydrophila strain S1 strain S1 stored in the refrigerator at 4°C with 200 ml of 5% sodium alginate solution on a vortex shaker, turn on the blower, and press Then turn on the spray dryer, peristaltic pump and needle pump, and set the parameters as follows: air volume display 100%, inlet air temperature 115°C, outlet air temperature 48°C, feed rate 35 ml / h, needle pass frequency 5 s. Maintain an air flow of 600 m during spray drying 3 / h. After the gas passes through the blower, it is filtered and heated to a set temperature of 115°C. The peristaltic pump sends the material solution to the sprayer, and the microcapsule particles are collected by the cyclone separator and stored in a refrigerator at 4°C.
Embodiment 3
[0034] Determination of Immunogenicity of Aeromonas hydrophila Microcapsule Vaccine
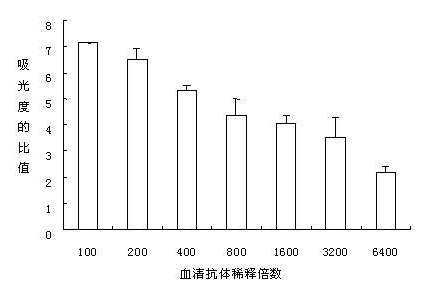
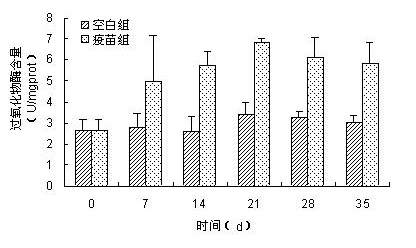
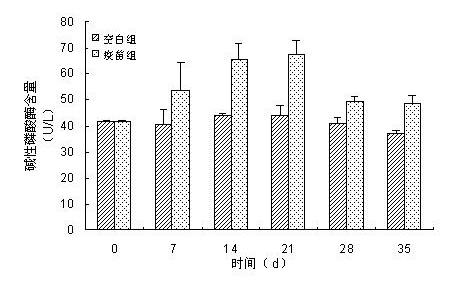
[0035] 1. Randomly select 30 healthy 4-week-old female BABL / C mice, and randomly divide them into two groups, that is, the Siberian sturgeon bacterial sepsis pathogenic Aeromonas hydrophila strain S1 microcapsule vaccine mixed oral group and the control group , each set up 3 parallel. Among them, the BABL / C mice of the Siberian sturgeon bacterial septicemia pathogenic Aeromonas hydrophila strain S1 microcapsule vaccine mixed with the oral group were fed with Siberian sturgeon bacterial sepsis pathogenic Aeromonas hydrophila strain S1 every day. The feed of the microcapsule vaccine, the dosage of the Siberian sturgeon bacterial septicemia pathogenic Aeromonas hydrophila strain S1 microcapsule vaccine was 6 g / Kg feed, and the BABL / C mice in the control group were fed without Siberian sturgeon bacteria every day The additive dose of S1 microcapsule vaccine of pathogenic Aeromonas hydrophila str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com