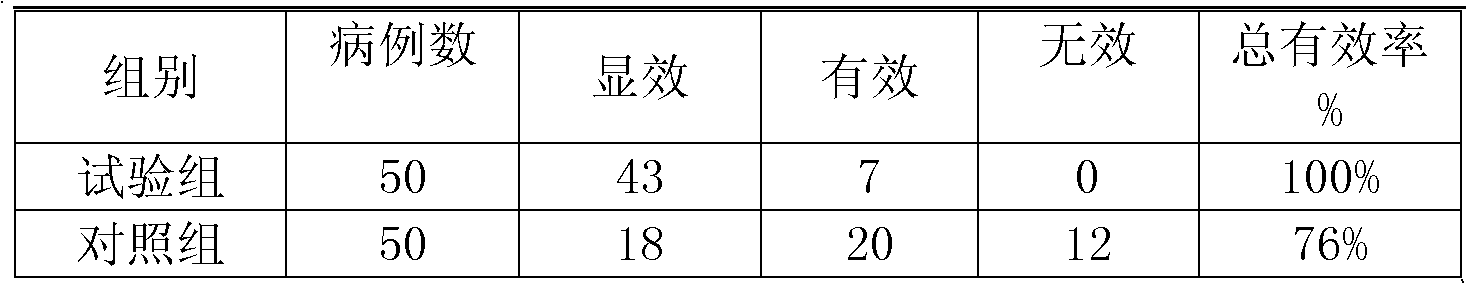
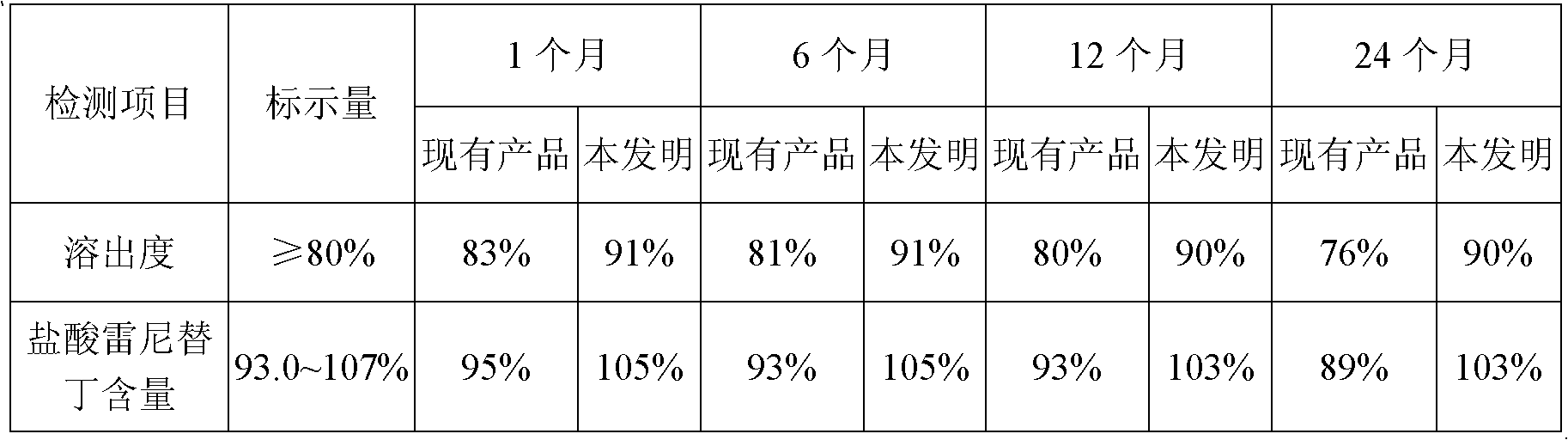
Ranitidine hydrochloride capsules and production method thereof
A technology of ranitidine hydrochloride and its production method, which is applied in the field of medicine for treating stomach diseases and its preparation, can solve the problems of different preparation processes, short storage time of medicines, weak stability, etc., achieve stable quality indicators, and solve the problem of hygroscopicity , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: a kind of ranitidine hydrochloride capsule, every 1000 capsules comprises the following components by weight: ranitidine hydrochloride 165g, calcium hydrogen phosphate 50g.
[0023] Weigh ranitidine hydrochloride and calcium hydrogen phosphate according to the parts by weight of the components; respectively pulverize ranitidine hydrochloride and calcium hydrogen phosphate and pass through 100 mesh sieves respectively, and add 50% ethanol in the tank mixer Stir and mix for 40 minutes to make soft materials and granulate; spread the prepared granules evenly on the baking tray with a thickness of 1-1.5cm, and then place the baking tray filled with granules in a hot air circulation drying oven at a temperature below 70°C The drying moisture is controlled at 3%; after drying, the drying car is pulled out from the oven and cooled to room temperature naturally, and the drying pan is taken out from the drying car (from bottom to top), and the granules in the pan ar...
Embodiment 2
[0024] Embodiment 2: a kind of ranitidine hydrochloride capsule, every 1000 capsules comprises the following components by weight: ranitidine hydrochloride 170g, calcium hydrogen phosphate 50g.
[0025] Weigh ranitidine hydrochloride and calcium hydrogen phosphate according to the parts by weight of the components; respectively pulverize ranitidine hydrochloride and calcium hydrogen phosphate and pass through 100 mesh sieves respectively, and add 50% ethanol in the tank mixer Stir and mix for 40 minutes to make soft materials and granulate; spread the prepared granules evenly on the baking tray with a thickness of 1-1.5cm, and then place the baking tray filled with granules in a hot air circulation drying oven at a temperature below 70°C The dry moisture is controlled within 4%; after drying, the oven is pulled out from the oven and cooled to room temperature naturally, and the oven tray is taken out from the oven (from bottom to top), and the granules in the tray are packed in...
Embodiment 3
[0026] Embodiment 3: a kind of ranitidine hydrochloride capsule, every 1000 capsules comprises the following components in parts by weight: ranitidine hydrochloride 167.4g, calcium hydrogen phosphate 43.1g.
[0027] Weigh ranitidine hydrochloride and calcium hydrogen phosphate according to the parts by weight of the components; respectively pulverize ranitidine hydrochloride and calcium hydrogen phosphate and pass through 100 mesh sieves respectively, and add 50% ethanol in the tank mixer Stir and mix for 20-40 minutes to make soft materials and granulate; lay the prepared granules evenly on the baking tray with a thickness of 1-1.5cm, and then place the baking tray filled with granules in a hot air circulation drying oven at 70 The dry moisture below ℃ is controlled within 4%; after drying, pull the drying car out of the oven to cool down to room temperature naturally, take out the drying pan from the drying car (from bottom to top), and put the granules in the pan into the tu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com